What is TriBi Minibody?
TriBi minibody is an innovative bispecific antibody that effectively recognizes two different targets simultaneously boosting tumor targeting and immune activation. Comprising three single-chain Fv (scFv) fragments, two of which bind to the tumor antigen HER2/neu and one to the CD16 receptor on NK cells, TriBi minibody activates both NK cells and tumor cells, thereby inducing antibody-dependent cellular cytotoxicity (ADCC). Compared to conventional monospecific or bispecific antibodies, TriBi minibody has higher affinity, lower dose, longer half-life and better stability.
Structure and Generation of TriBi Minibody
The basic structure of TriBi minibody consists of an IgG1 CH3 constant domain functioning as the oligomerization domain, connecting two different scFvs. One of the scFvs binds to the tumor antigen HER2/neu and the other scFv binds to the CD16 receptor on NK cells. This structure enables TriBi minibody to recognize two different targets simultaneously, resulting in superior tumor targeting and immune activation.
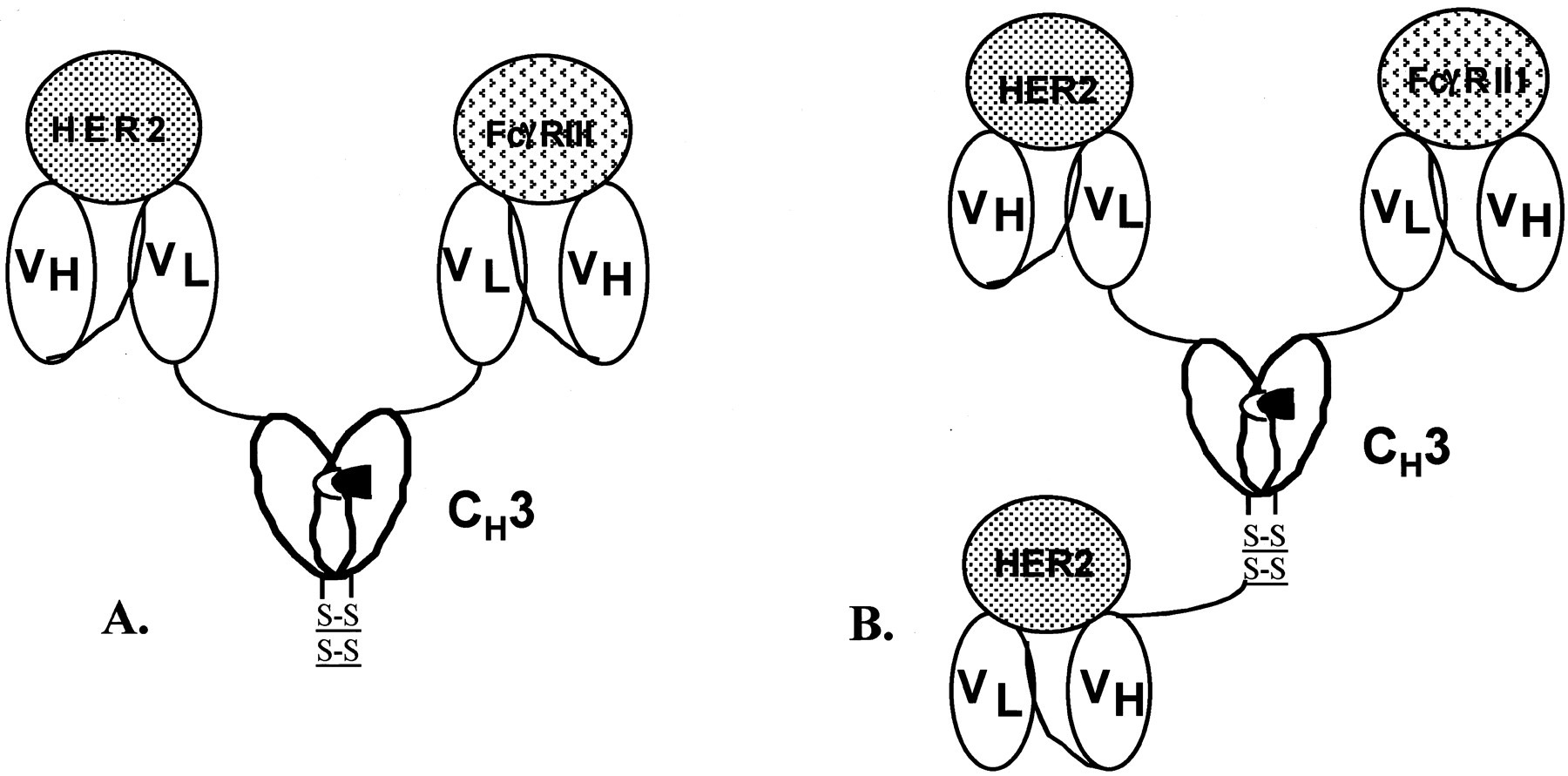
Fig.1 Schematic Diagram of Minibody Construction (Shahied LS, 2004)
TriBi minibody can be efficiently expressed in mammalian cells from a bicistronic vector and subsequently purified through sequential affinity chromatography. To enhance heterodimer formation, "knobs and holes" structures can be created through single-residue mutations in the CH3 domains. Moreover, additional cysteine residues can be introduced to stabilize the bispecific minibody structure.
Optimization of TriBi minibody involves modifying the length, angle, and orientation of the linkers between scFvs to improve their binding properties and functions. Shorter linkers generally increase scFv interaction, while longer linkers enhance flexibility. The angle and orientation of linkers also influence spatial arrangement and steric hindrance between scFvs. Precise tuning of these parameters results in TriBi minibodies with optimal affinity, stability and functionality.
Advantages and Disadvantages of Tribi Minibody
TriBi minibody exhibits a range of advantages and disadvantages, which are contingent upon on its structure, function, and application. Notably, TriBi minibody's capacity to simultaneously recognize two targets leads to enhanced tumor targeting and immune activation, enabling effective tumor lysis at lower concentrations. Additionally, it maintains long-term stability in vivo and can be prepared using straightforward expression and purification techniques. However, TriBi minibody's disadvantages stem from its potential to provoke immunogenic reactions, leading to antibody neutralization or allergic responses. It may also cross-react with non-specific targets, resulting in off-target tissue damage or adverse reactions. Furthermore, its efficacy and safety can be influenced by factors within tumor microenvironment.
Clinical Data of Tribi Minibody
The clinical data of TriBi minibody mainly include two parts: the approved one and the one in clinical trials. Currently, there is one approved TriBi minibody called Margetuximab (MGAH22). This antibody targets HER2/neu and CD16a and is used in the treatment of patients with HER2-positive advanced breast cancer. Margetuximab's approval was based on a randomized, double-blind, multicenter phase III clinical trial (SOPHIA) comparing its efficacy and safety to Trastuzumab combined with chemotherapy in patients with HER2-positive advanced breast cancer. The results of SOPHIA trial showed that the Margetuximab combined with chemotherapy resulted in longer progression-free survival (PFS) and overall survival (OS) compared to the Trastuzumab combined with chemotherapy group, with no significant difference in objective response rate (ORR) and adverse events (AEs) between the two groups.
Apart from Margetuximab, several other TriBi minibodies are currently undergoing clinical trials, mainly targeting different tumor antigens and immune effectors.
Table 1. TriBi Minibodies in Different Stages of Clinical Trials
|
TriBi Minibody
|
Targets
|
Tumor types
|
Clinical trial phase
|
Clinical trial ID
|
|
MGC018
|
B7-H3 and CD16a
|
B7-H3-positive solid tumors
|
Phase I
|
NCT03729596
|
|
MGC019
|
PD-L1 and CD16a
|
PD-L1-positive solid tumors or lymphomas
|
Phase I
|
NCT03905135
|
|
MGC020
|
CD20 and CD16a
|
CD20-positive B-cell malignancies
|
Phase I
|
NCT04105094
|
|
MGC021
|
CEA and CD16a
|
CEA-positive solid tumors
|
Phase I
|
NCT04123470
|
|
MGC022
|
PSMA and CD16a
|
PSMA-positive prostate cancer
|
Phase II
|
NCT03706365
|
References
1. Cuesta AM, et al. Multivalent antibodies: when design surpasses evolution. Trends Biotechnol. 2010;28(7):355-62.
2. Nuñez-Prado N, et al. The coming of age of engineered multivalent antibodies. Drug Discov Today. 2015;20(5):588-94.
3. Shahied LS, et al. Bispecific minibodies targeting HER2/neu and CD16 exhibit improved tumor lysis when placed in a divalent tumor antigen binding format. J Biol Chem. 2004;279(52):53907-14.
4. Xu Y, et al. Production of bispecific antibodies in "knobs-into-holes" using a cell-free expression system. MAbs. 2015;7(1):231-42.
5. Rugo HS, et al. SOPHIA primary analysis: A phase 3 (P3) study of margetuximab (M) + chemotherapy © versus trastuzumab (T) + C in patients (pts) with HER2+ metastatic (met) breast cancer (MBC) after prior anti-HER2 therapies (Tx). J Clin Oncol. 2019;37(15_suppl):1000.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY










