Background of Diabody
Bispecific antibodies (BsAbs) are a class of antibody molecules that can simultaneously recognize two different antigens or epitopes, with functions such as expanding antigen recognition range, enhancing signal transduction, and achieving multiple targeting. Since the first BsAb was prepared in 1985, BsAbs have become a hot topic in the field of antibody engineering, and various formats of BsAbs have been designed and developed for diagnosis and treatment of various diseases, especially cancer. Diabody is a bispecific antibody fragment composed of two antigen-binding Fv domains. They are one of hundreds of different formats of BsAbs because they are small and rigid enough to be crystallized. Compared with other BsAb formats, Diabody has many advantages, such as high affinity, high stability, high permeability, low immunogenicity and easy preparation, which make it have a wide range of application potential in diagnosis and treatment.
Structural Characteristics of Diabody
Diabody is a bispecific antibody fragment composed of two antigen-binding Fv domains. Each Fv domain consists of a heavy chain variable region (VH) and a light chain variable region (VL), which are connected by a short peptide linker. Unlike single-chain Fv (scFv), each antigen-binding site of Diabody is formed by pairing of VH and VL domains from two different polypeptides. The two polypeptides of Diabody form a stable dimer through non-covalent interactions between the VH domains, with a size of about 50 kDa.
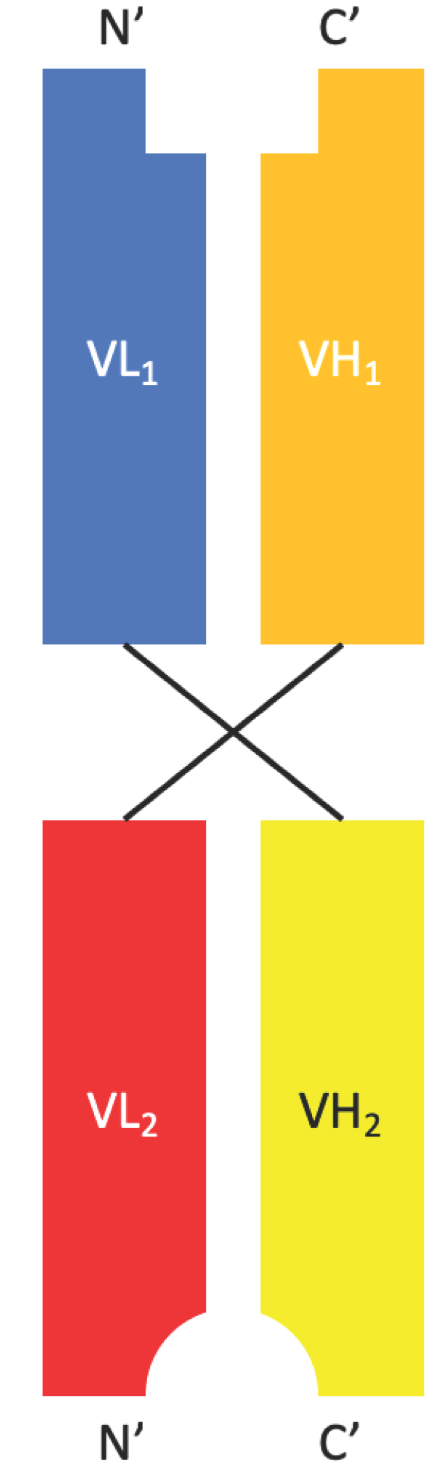
Fig.1 Schematic structure of Diabody. (Bates A, 2019)
Generation Methods of Diabody
Diabody can be generated by various methods, such as genetic engineering, phage display, yeast display, etc. Each method has its own advantages and disadvantages and applicability. The main methods are summarized as follows:
-
Genetic engineering: This method involves cloning the genes encoding the V H and V L domains of two different antibodies into a single expression vector, with a short linker (usually 5 amino acids) between them. The vector is then transformed into E. coli or other host cells for expression and purification of Diabody. This method is simple and efficient, but it may result in low expression level, misfolding or aggregation of Diabody.
-
Phage display: This method involves constructing a phage display library of Diabody by fusing the genes encoding the V H and V L domains of two different antibodies with a phage coat protein gene, such as pIII or pVIII. The library is then screened for binding to the desired antigens by panning or affinity selection. This method allows for high-throughput selection and optimization of Diabody with high specificity and affinity.
-
Yeast display: This method involves displaying Diabody on the surface of yeast cells by fusing the genes encoding the V H and V L domains of two different antibodies with a yeast surface protein gene, such as Aga2p or Sed1p. The yeast cells are then screened for binding to the desired antigens by fluorescence-activated cell sorting (FACS) or magnetic-activated cell sorting (MACS). This method offers advantages such as eukaryotic expression system, post-translational modifications and glycosylation of Diabody.
Other methods for generating Diabody include bacterial display, ribosome display, mammalian cell display, etc. These methods have their own merits and limitations and can be used for different purposes and applications.
Diabody in Clinical Applications
Diabody, as a bispecific antibody format, has advantages such as high affinity, high stability, high penetration and low immunogenicity, making it a promising anti-tumor immunotherapy strategy. Currently, several Diabodies have entered clinical trials or approved for marketing, mainly targeting tumor-associated antigens or immune checkpoint molecules.
Currently, only one Diabody has been approved by the US Food and Drug Administration (FDA), which is Blinatumomab (Blincyto) targeting CD19 and CD3. The drug was granted accelerated approval in December 2014 for the treatment of relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL). In July 2017, the drug was granted full approval and expanded its indications to include children and adolescents. The drug works by connecting CD19-positive B cells with CD3-positive T cells, activating the killing function of T cells and thereby eliminating B cells. The clinical trial results of the drug showed that it can significantly improve the complete remission rate and disease-free survival of B-ALL patients.
In addition to Blinatumomab, there are some Diabodies undergoing clinical trials, mainly targeting different tumor-associated antigens or immune checkpoint molecules. The common feature of these Diabodies is that they use CD3 as a T cell inducer to guide T cells to the surface of tumor cells, thereby achieving tumor-specific killing. Among them, REGN1979 is the fastest progressing Diabody, which has entered phase II clinical trials, targeting CD20-positive B-cell malignancies. The results of its phase I clinical trial showed that it has good tolerability and significant anti-tumor activity in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL).
Table 1. some Diabodies in clinical trials and their related information
|
Diabody
|
Target
|
Indication
|
Clinical trial phase
|
Main institution
|
|
PF-07062119
|
EGFRvIII/CD3
|
Glioblastoma
|
Phase I
|
Pfizer
|
|
PEG-AVP0458
|
TAG-72/CD3
|
Prostate cancer, ovarian cancer
|
Phase I
|
Avipep
|
|
REGN4018
|
MUC16/CD3
|
Ovarian cancer
|
Phase I
|
Regeneron
|
|
REGN5459
|
BCMA/CD3
|
Multiple myeloma
|
Phase I
|
Regeneron
|
|
REGN5678
|
PSMA/CD28
|
Prostate cancer
|
Phase I
|
Regeneron
|
|
REGN1979
|
CD20/CD3
|
Non-Hodgkin lymphoma, chronic lymphocytic leukemia
|
Phase II
|
Regeneron
|
In summary, Diabody as a novel bispecific antibody format shows high potential and prospect in clinical applications. However, Diabody also faces some challenges and limitations, such as dose selection, toxicity management, immunogenicity control, etc. Therefore, further optimization of Diabody design and preparation is needed, as well as more clinical trials to verify its safety and efficacy, and bring benefits to more cancer patients.
References
1. Scott AM, et al. First clinical study of a pegylated diabody 124I-labeled PEG-AVP0458 in patients with tumor-associated glycoprotein 72 positive cancers. Theranostics. 2020 Sep 15;10(25):11404-11415.
2. Kato Y, et al. Generation and characterization of a bispecific diabody targeting both EPH receptor A10 and CD3. Biochem Biophys Res Commun. 2015 Jan 24;456(4):834-40.
3. Seifert O, et al. Diabody-Ig: a novel platform for the generation of multivalent and multispecific antibody molecules. Antibody Therapeutics. 2019 Jul;11(5):919-929.
4. Bates A, et al. David vs. Goliath: The Structure, Function, and Clinical Prospects of Antibody Fragments. Antibodies (Basel). 2019 Apr 9;8(2):28.
5. Holliger P, et al. Diabodies: small bivalent and bispecific antibody fragments. Proc Natl Acad Sci U S A. 1993 Jul 1;90(14):6444-8.
6. Lu D, et al. Construction and characterization of tetravalent bispecific di-diabodies using the C H 3 domain as a dimerization motif. Protein Eng Des Sel. 2003 Dec;16(12):1047-54.
7. Asano R, et al. A humanized anti-EGFR antibody conjugated to anti-CD3 activates T cells and induces specific tumor lysis. Cancer Immunol Immunother. 2006 Oct;55(10):1237-47.
8. Stork R, et al. A trifunctional bispecific diabody that mediates T cell activation and tumor cell lysis through binding to CD3 and carcinoembryonic antigen (CEA). J Immunother. 2007 Nov-Dec;30(8):798-808.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY










