Immuno-PCR combines the exponential signal amplification power of PCR with the versatility and flexibility of ELISA and offers great promise for its application in various aspects of biological and medical sciences, as well as clinical diagnostics and forensic medicine. Methods based on Immuno-PCR have been developed in Creative Biolabs. We also offer other ELISA kits for specific detection of disease-related proteins. Our professional team is optimized to help you with high-quality and cost-effective testing service to make your project a success.
Immuno-PCR is an antigen detection system in which polymerase chain reaction (PCR) is used to amplify labeled DNA fragments that specifically attach to antigen-antibody complexes. Since ELISA requires the use of antibodies, it is suitable for any protein but its sensitivity is not sufficient for the detection of analytes of low abundance. Due to the enormous amplification capacity and specificity of PCR, immune-PCR allows the detection sensitivity of specific antigens to be significantly enhanced compared to conventional antigen detection systems such as ELISA. It combines the specificity of ELISA and signal amplification of PCR. The superiority and versatility of immune PCR, including high sensitivity and easy control of contamination, provide great promise for applications in biology, medical science, clinical diagnosis, and forensic medicine.
 Fig.1 A schematic representation of PCR-ELISA and immuno-PCR.1,5
Fig.1 A schematic representation of PCR-ELISA and immuno-PCR.1,5
Biomolecular detection, particularly at the single cell or even single molecule level, has become extremely important for a variety of purposes, including disease diagnosis. Immunoassays based on the interaction between antigens and antibodies are the most common and fundamental methods used to detect target proteins from relatively large amounts of body liquid or tissue. However, many of these methods have sensitivity limits that curtail their use in modern laboratory diagnostic procedures. PCR can detect extremely low copy numbers of DNA, which satisfies the demand for highly sensitive, fast, and cost-efficient nucleic acid detection. Creative Biolabs has been devoted for the development of highly sensitive, more flexible, quantitative, and easy-to-use Immuno-PCR based Kits, which combines the exponential signal amplification power of PCR with the versatility and flexibility of ELISA.
 Fig.2 Result of immuno-PCR.2
Fig.2 Result of immuno-PCR.2
Creative Biolabs is a world leader in the development of ELISA based kits and now offers Immuno-PCR based kits for specific detection of disease-related proteins. If you are interested in our ELISA kits development, please feel free to contact us for more details.
1. Ultrasensitive Immuno-PCR for Crustacean Tropomyosin Detection
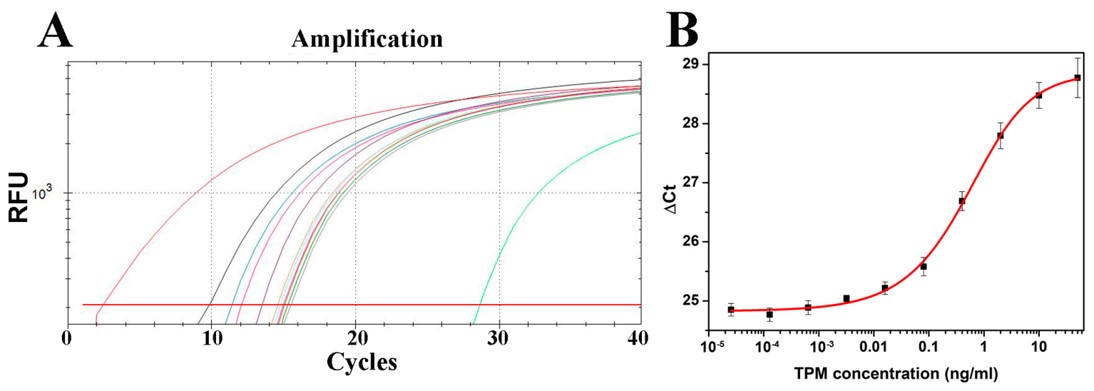 Fig.3 Crustacean TPM quantification results detected by immuno-PCR.3,5
Fig.3 Crustacean TPM quantification results detected by immuno-PCR.3,5
This study presented an ultrasensitive immuno-PCR method for quantifying crustacean tropomyosin (TPM), a major food allergen. The method combined sandwich ELISA and real-time PCR (rtPCR) for the amplification and detection of marker DNAs. A monoclonal anti-TPM antibody served as the capture antibody, while a polyclonal rabbit anti-shrimp tropomyosin antibody was used for detection, with natural shrimp TPM as the standard. A double-stranded amino-DNA was linked to an anti-rabbit secondary antibody and subsequently amplified through rtPCR. The immuno-PCR method offered 20-fold higher sensitivity than traditional ELISA, with a LOQ of 19.8 pg/mL. This assay was highly specific and precise across a broad concentration range. As the first immuno-PCR assay for food allergens, it provides a powerful tool for detecting trace amounts of TPM and could be adapted for the ultrasensitive detection of other food allergens currently quantified by ELISA.
2. Covalent and Cleavable Antibody-DNA Conjugates for Sensitive Immuno-PCR
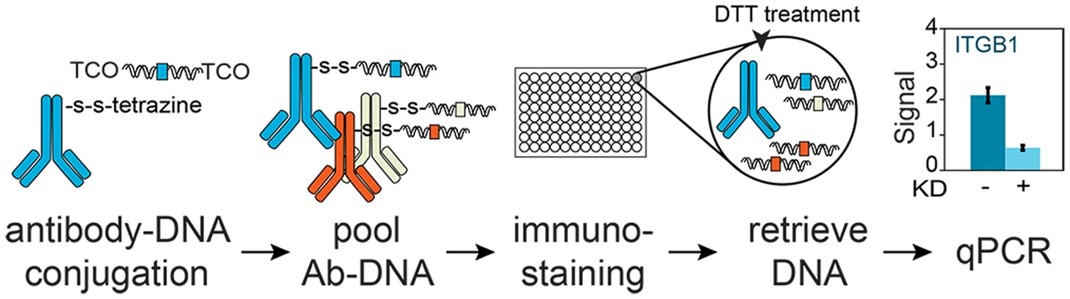 Fig.4 Overview of Immuno-PCR method using antibody-dsDNA conjugates.4,5
Fig.4 Overview of Immuno-PCR method using antibody-dsDNA conjugates.4,5
In this study, researchers developed a strategy for conjugating antibodies with double-stranded DNA (dsDNA) for sensitive immuno-PCR. The approach included a simple antibody functionalization step and a two-step dsDNA functionalization, followed by conjugation using tetrazine and trans-cyclooctene (TCO). A dithiothreitol (DTT)-cleavable linker enabled the release of dsDNA after immuno-staining for sensitive detection via qPCR. Different dsDNA sequences could be conjugated to antibodies, enabling multiplexed immuno-PCR. The strategy's throughput could be increased through parallel, miniaturized, or automated reactions. This chemically cleavable antibody-DNA conjugates will enable the development of multiplexed immuno-PCR and immuno-sequencing techniques.
References
For Research Use Only.
