Based on its good prognostic value and potential usefulness as an antigen, prostatic acid phosphatase (PAP) has become a valuable biomarker in prostate cancer. As a world leader in the antibody market, Creative Biolabs has established a suite of in vitro diagnostic (IVD) antibody development services for PAP detection. As well, our team is committed to designing and exploiting antibodies against various markers to improve the diagnosis, treatment, and prognosis of various diseases.
PAP, also known as acid phosphatase, prostate (ACPP), is a 100-kDa secreted glycoprotein enzyme that is synthesized under androgen regulation and is secreted by the prostate gland’s epithelial cells. The PAP monomer has six conserved cysteine (Cys) residues that constitute three disulfide bonds (Cys129-Cys340, Cys183-Cys281, and Cys315-Cys319) as well as three putative N-linked glycosylation sites. These active sites and glycosylation sites are conserved in all mammalians. PAP is a type of non-specific phosphomonoesterase that displays phosphatase activity in acidic conditions of pH 4-6 as its name suggests. This enzyme can dephosphorylate a number of substrates including alkyl, aryl, acyl orthophosphate monoesters, and phosphorylated proteins. Also, PAP has lipid phosphatase activity and could inactivate lysophosphatidic acid in seminal plasma.
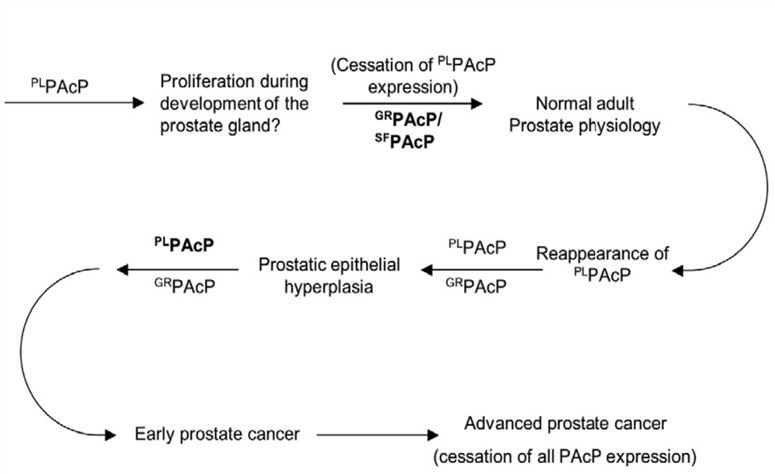 Fig.1 Proposed role of PAcP conformers in the natural history of prostate cancer.1
Fig.1 Proposed role of PAcP conformers in the natural history of prostate cancer.1
Prostate cancer is one of the most prevalent non-skin cancers and the second cause of cancer deaths among males in Western countries. There is a higher probability that prostate cancer will be completely cured If it’s diagnosed in early stages. Notably, PAP has emerged as the first clinically useful tumor marker in the 1940s.
PAP is identified in numerous organs such as the brain, liver, and lung, while the highest concentration shown in the prostate. Previous studies indicated that the expression of secretory PAP proportionally increases with prostate cancer progression. And high-level PAP was detected in high Gleason score prostate cancers as analyzed by immunohistochemistry (IHC). In another case, cancer-specific survival assays with 193 patients’ serum revealed that when PAP is <1.5 U/L, 1.5-2.4 U/L, and >2.5 U/L, the progression of prostate cancer is 93%, 87%, and 75% (p=0.013), respectively. Hence, PAP is a biochemical diagnostic mainstay, routinely used in the diagnosing and staging of prostate cancer. Furthermore, it appears to be extremely valuable in predicting distant failure in intermediate- and high-risk patients, for which significant controls are achieved with aggressive initial local treatment.
Despite the great progress in understanding the disease process and diagnostic criteria for prostate cancer, the majority of patients are identified at early stages with an uncertain prognosis. Recently, there’s a renewed interest in PAP marker because of its largely higher correlation with prostate cancer progression and its success in the immunotherapy of prostate cancer. At Creative Biolabs, we provide customized IVD antibody development services targeting PAP. Based on our advanced IVD platform, we also offer professional immunoassay development service for different diagnostic markers. Our immunoassay kit development service is based on different methods, including ELISA, lateral flow, turbidimetric immunoassay, etc.
Creative Biolabs is skilled at offering one-stop, customized services for the development of biomarker-specific antibody for global clients. If you’re interested in our services, please contact us for more information or directly send us an inquiry.
Reference
For Research Use Only.
