In Vitro SSP Study on Autonomic Nervous System
Creative Biolabs is an expert in the field of drug discovery and assessment. As part of an IND-enabling program, safety pharmacology studies are an essential step in assessing acute and potentially life-threatening risks of novel pharmaceuticals. Now Creative Biolabs provides the in vitro supplemental safety pharmacology studies on the autonomic nervous system.
Introduction to Supplemental Safety Pharmacology (SSP) Studies
Safety Pharmacology studies are defined to investigate the potential undesirable pharmacodynamic effects of new chemical entities (NCEs) on physiological functions in relation to exposure in the therapeutic range and above. In addition to the main vital systems (Central Nervous System, Cardiovascular System, and Respiratory System), the supplemental studies are always performed to evaluate potential adverse pharmacodynamics effects on organ system functions not addressed by the core battery. Here, we mainly focus on the effects of the test substance on the autonomic nervous system.
Introduction to Autonomic Nervous System
The autonomic nervous system (ANS), formerly the vegetative nervous system, is a network of nerves and ganglia that controls the involuntary physiologic actions and maintains internal homeostasis. The autonomic nervous system is composed of three branches which are the sympathetic nervous system, the parasympathetic nervous system, and the enteric nervous system. This system has been served as the primary mechanism in control of the fight-or-flight response. The autonomic nervous travel to organs throughout the human body include stomach, duodenum, jejunum, ileum, spleen, gallbladder, liver, colon, pancreatic head, appendix, kidneys, and ureters.
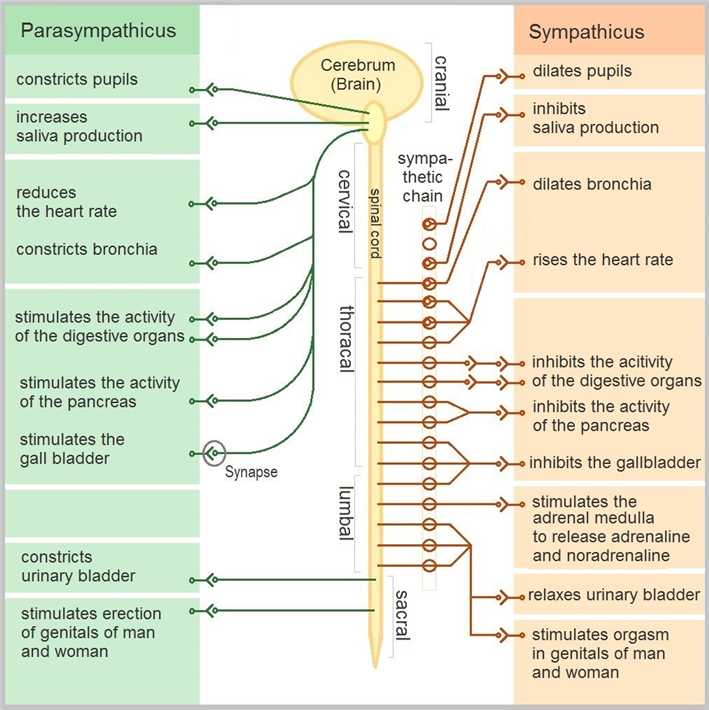 Fig.1 The innervation of autonomic nervous system (ANS).
Fig.1 The innervation of autonomic nervous system (ANS).
In Vitro SSP Studies on Autonomic Nervous System
For the supplemental safety pharmacology studies, here we focus on the effects of the test substance on the autonomic nervous system. As components of autonomic nerves, sympathetic and parasympathetic nerves have dual dominance and alternating excitatory relationships in controlling the body, while sympathetic nerves are more dominant in governing activities of the cardiovascular system, while in the pupil, smooth muscle, and glands, the parasympathetic nerve is in a comparative advantage place. The targets where drugs exert neurotoxic effects are often the basic life-active substances in the system, such as precursors of neurotransmitters, synthetases, storage vesicles, related factors for the uptake and release of transmitters, receptors, and inactivation and degradation enzymes of transmitters, as well as decomposition products of the transmitter.
As targets of neurotoxicity, synapses undergo compensatory phenomena after injury, which are manifested by changes in the number of receptors and enzyme activities, which are also part of the mechanism of neurotoxicity. Therefore, the study of the neurotoxicity of drugs is basically carried out from the content of the process of synaptic transmission. Scientists from Creative Biolabs use a combination of morphology, physiology, electrophysiology, biochemistry, molecular biology, and brain imaging to conduct a comprehensive or customized study of the neurotoxic effects of drugs. Based on the characteristics of the drug and the purpose of clinical use, we can select test methods flexibly according to models of pharmacodynamics response, characteristics of pharmacokinetics.
Partial Strategies for Neurosafety Study
- Safety studies targeting receptors (G-protein-mediated receptors, ion channel-coupled gated receptors, allosteric site voltage-gated receptors, intracellular steroid receptors)
- Safety studies targeting cell signaling (Ca2+ concentration, cAMP and cGMP)
- Safety study targeting glial cells
- Morphological method
- Electrophysiological method (electrophysiology, patch clamp, etc.)
- Biochemical method (lysosomal enzyme, luciferase, β-galactosidase, acid phosphatase, and other indicators)
Features
- Extensive professionalism and experience
- Advanced biological platforms
- Fully customizable experimental design to expand beyond standard procedure
- Competitive price with the best quality
Following the International Conference on Harmonisation (ICH) Guidelines (S7A, S7B), our experienced scientists are confident in offering the best services to advance your program successfully. If you are interested in our services, please do not hesitate to contact us for more details.
For Research Use Only.
