ADEPT Acute and Chronic Toxicity Testing Service
Introduction Services Case Study Features
To ensure the safety and efficacy of these novel medications, Creative Biolabs provides a full range of preclinical acute and chronic toxicity testing services to support your research and development efforts in the Antibody-Directed Enzyme Prodrug Therapy (ADEPT) field.
Introduction
Preclinical acute and chronic toxicity testing services provide an effective means to evaluate potential toxic reactions in new antibody drugs. Currently, Creative Biolabs systematically assesses the impact of antibody drugs on living organisms over both short and long terms. This not only identifies the safe dosage range but also reveals possible side effects. Furthermore, our acute and chronic toxicity testing services can facilitate drug optimization and enhancement, assisting clients in confirming the efficacy and safety of their products at early stages, and ultimately supporting the successful launch of innovative ADEPT treatment solutions.
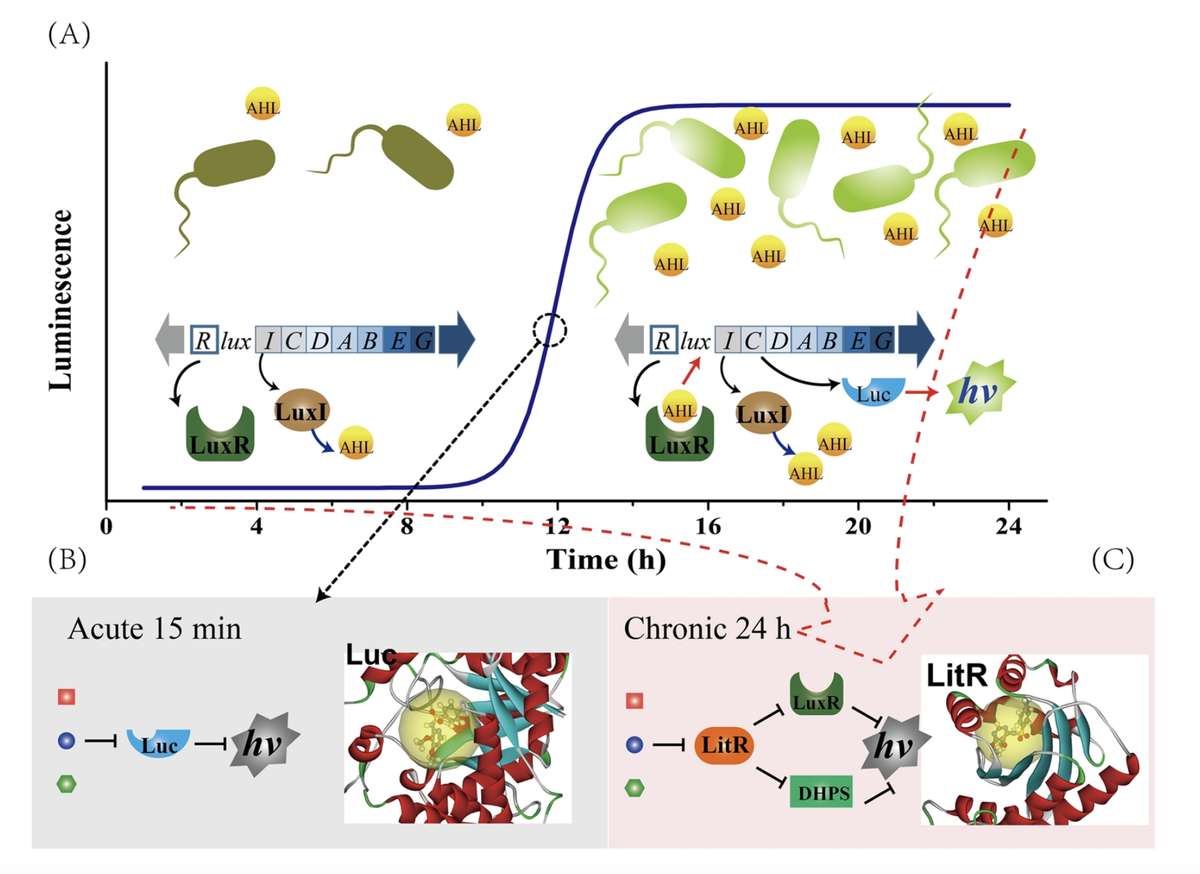 Fig.1 The MOA of Acute and Chronic Toxicity.1,3
Fig.1 The MOA of Acute and Chronic Toxicity.1,3
Services
Creative Biolabs offers preclinical acute and chronic toxicity testing services to enhance your understanding of the safety profile of antibody drugs under various conditions. With our expert testing platforms and extensive experience, we provide precise and reliable toxicity assessments, helping you mitigate potential risks during the research and development process.
-
Acute Toxicity Testing Services
-
Assessment of Acute Toxicity:
- Utilization of small animal models (such as mice and rats) to observe the systemic effects of drugs over a short duration.
- Evaluation of dose-response relationships to determine the median lethal dose (LD50) while monitoring primary toxic manifestations.
-
Evaluation of Organ Damage:
- Conduct biochemical tests to assess liver and kidney function and evaluate the extent of multi-organ injuries.
- Perform histopathological examinations through tissue slicing to observe changes in cellular morphology, aiding in the identification of toxic mechanisms.
-
Kinetic Analysis:
- Investigate the metabolic processes of drugs within the body, utilizing pharmacokinetic models to provide scientific support for toxicity assessments.
-
Chronic Toxicity Testing Services
-
Long-Term Administration Studies:
- Employ chronic dosing models to assess the toxic effects of drugs under prolonged usage.
- Monitor physiological parameters, weight changes, and behavioral evaluations in animals to obtain comprehensive safety data.
-
Multi-Generation Reproductive Studies:
- Perform assessments of reproductive toxicity, focusing on the drug's impact on the reproductive system and the developmental outcomes of offspring.
- Explore potential genetic toxicity to ensure that long-term drug use does not adversely affect future generations.
-
Accumulated Toxicity Research:
- Assess compound accumulation effects and long-term safety through periodic monitoring and regular biochemical evaluations.
 Fig.2 Toxicity Assays for Acute and Chronic Toxicity Testing.2,3
Fig.2 Toxicity Assays for Acute and Chronic Toxicity Testing.2,3
Case Study: ADEPT Acute and Chronic Toxicity Testing
a. PARTI: Acute Toxicity Assessment
Animal Selection: Utilize healthy C57BL/6 mice, dividing them into an experimental group and a control group, with 10 mice in each group.
Dose Selection: Based on the results from preliminary antibody drug screening, identify three different dosage levels (low, medium, high) for evaluation.
Administration Method: Administer the antibody drug to the mice via intravenous injection (IV), monitoring toxic reactions within 24 hours post-administration.
Observation Parameters:
Behavioral Changes (such as activity levels and weight fluctuations)
Physiological Measurements (temperature and respiratory rate)
Pathological Examination, focuses primarily on the liver, kidneys, and other vital organs.
b. PART II: Chronic Toxicity Assessment
Experimental Design: Select healthy Sprague-Dawley (SD) rats, dividing them into an experimental group (receiving long-term treatment) and a control group.
Dose and Administration: Determine an appropriate dose (set at one-tenth of the high dose used in the acute toxicity study) and administer it continuously for 12 weeks, three times per week.
Monitoring Parameters:
Drug concentration is measured in milligrams per kilogram of body weight;
Routine blood biochemistry indicators (evaluating liver and kidney function);
Histopathological examinations, with particular attention to the long-term effects on visceral organs.
c. PART III: Data Analysis
Within 24 hours post-administration, the high-dose group exhibited a mortality rate of 20%, while no significant responses were noted in the low-dose group.
Behavioral observations indicated that mice in the high-dose group displayed marked ataxia and a loss of body weight.
The body weight trend in the long-term treatment group remained relatively stable, showing no significant difference when compared to the control group (P>0.05).
Biochemical analysis of blood revealed that liver function indicators (such as ALT and AST) did not show any noticeable increase after six months of treatment, suggesting that the liver was not significantly affected.
Pathological evaluation revealed no significant morphological changes in liver tissue among the drug-treated group, with cellular structures remaining intact.
Features
-
Comprehensive Toxicity Assessment: Creative Biolabs offers an extensive range of toxicity testing protocols, including evaluations for acute, subacute, and chronic toxicity. Our systematic approach allows for a thorough examination of potential toxic reactions induced by various dosages over different periods.
-
Diverse Animal Models: To more accurately replicate human physiological responses, Creative Biolabs uses a variety of animal models such as mice, rats, and dogs. We select the most appropriate subjects based on the specific characteristics of the drug, ensuring the reliability and translatability of the results.
-
Tailored Services: In alignment with the unique attributes of different ADEPT candidate drugs and their development needs, we provide customized experimental designs and testing plans. This ensures compliance with specific regulatory requirements and facilitates the achievement of scientific goals.
-
Comprehensive Data Analysis: Beyond standard toxicity assays, Creative Biolabs offers data analysis, statistical support, and report generation services. This enables research teams to thoroughly interpret experimental results, providing a solid scientific foundation for subsequent decision-making.
-
Rapid Turnaround: Creative Biolabs is typically equipped with efficient experimental processes and ample resource allocation, allowing them to quickly address client needs, reduce research timelines, and assist in the swift transition of products into the clinical stage.
At Creative Biolabs, our toxicity testing services feature a comprehensive assessment framework designed to accurately evaluate the potential effects of new drugs on biological systems. Whether you require acute toxicity testing to quickly gauge a drug's initial safety or long-term chronic toxicity testing to ensure its safety during prolonged use, our expert team is committed to delivering efficient and precise results. We invite you to contact us for more information about our preclinical acute and chronic toxicity testing services. We look forward to collaborating with you on advanced ADEPT innovation and development.
References
-
Wang, Dali, et al. "A mechanism-based QSTR model for acute to chronic toxicity extrapolation: a case study of antibiotics on luminous bacteria." Scientific Reports 7.1 (2017): 6022.
-
Xiong, Huajiang, Catherine Pears, and Alison Woollard. "An enhanced C. eelegans-based platform for toxicity assessment." Scientific rReports7.1 (2017): 9839.
-
Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only | Not For Clinical Use


 Fig.1 The MOA of Acute and Chronic Toxicity.1,3
Fig.1 The MOA of Acute and Chronic Toxicity.1,3
 Fig.2 Toxicity Assays for Acute and Chronic Toxicity Testing.2,3
Fig.2 Toxicity Assays for Acute and Chronic Toxicity Testing.2,3
 Download our brochure
Download our brochure