Constitutive Androstane Receptor Isoform 3 (CAR3) Nuclear Receptor Assay Service
Precision in Every Step: Unleash CAR3's Potential with Our Expert Assay Services
Constitutive androstane receptor three (CAR3), is a variation of the nuclear receptor CAR, that is more often than not concerned with the regulation of drug metabolism and detoxification pathways within the liver. CAR3 is thought for its constitutive interest, which means it's miles lively even in the absence of a ligand. This makes it a vast target for analyzing drug-drug interactions, xenobiotic metabolism, and liver-precise gene regulation. Nuclear receptor assay services are designed to observe and compare the pastime, binding affinity, and functionality of nuclear receptors. These assays may be used to screen for ability drug applicants, look into receptor-ligand interactions, and understand the molecular mechanisms underlying numerous sicknesses.
The CAR3 nuclear receptor assay is essential in the study of absorption, distribution, metabolism, and excretion (ADME) techniques. Creative Biolabs is at the forefront of biotechnological innovation, offering specialized assay offerings to boost drug discovery and development. Our CAR3 nuclear receptor assay is meticulously designed to offer complete insights into the activity and regulation of the CAR3 receptor. By leveraging advanced technologies and a crew of professional scientists, Creative Biolabs supplies wonderful, reproducible facts that support the identification and optimization of novel therapeutics.
-
High-throughput screening: Rapid identification of potential drug candidates that interact with CAR3.
-
Binding affinity assays: Detailed analysis of ligand-receptor interactions to determine binding strengths and specificities.
-
Functional assays: Evaluation of CAR3 activity in response to various compounds, providing insights into the receptor's role in drug metabolism.
Our service is designed to provide unparalleled precision and reliability, ensuring that researchers and pharmaceutical developers receive the highest quality data for their projects. With a team of dedicated scientists and state-of-the-art facilities, Creative Biolabs is committed to advancing the field of drug discovery and development through innovative and customized assay solutions.
Our CAR3 nuclear receptor assay service features a robust and reliable methodology. We utilize human CAR3 receptors in a functional, cell-based assay format to evaluate both agonist and antagonist activities. The detection of functional responses is quantified through IC50 measurements, providing detailed insights into the potency and efficacy of test compounds.
|
Receptor
|
Assay Type
|
Subtype
|
Functional Mode
|
Detection
|
|
Human CAR3
|
Functional
|
Cell-based
|
Agonist
|
IC50
|
|
Functional
|
Cell-based
|
Antagonist
|
IC50
|
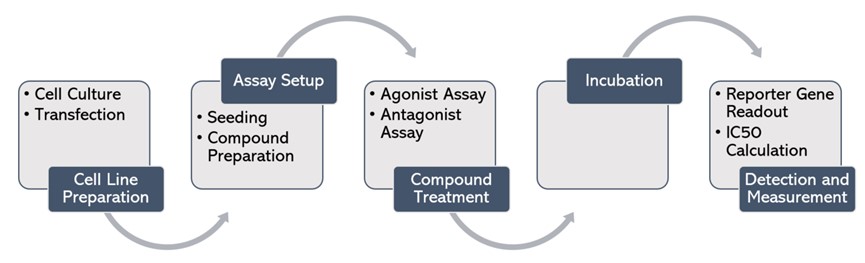
Technical Workflow
-
Cell Line Preparation
-
Cell Culture: Cells expressing the human CAR3 receptor are cultured under optimal conditions.
-
Transfection: Cells are transfected with a reporter gene construct designed to provide measurable outputs upon receptor activation.
-
Assay Setup
-
Seeding: Transfected cells are seeded into 96-well or 384-well plates to allow for high-throughput screening.
-
Compound Preparation: Test compounds, including both potential agonists and antagonists, are prepared at varying concentrations for dose-response analysis.
-
Compound Treatment
-
Agonist Assay: Cells are treated with test compounds to assess agonist activity, measuring the extent to which each compound activates the CAR3 receptor.
-
Antagonist Assay: Cells are pre-treated with known CAR3 agonists followed by test compounds to assess the antagonist activity, determining how effectively each compound inhibits receptor activation.
-
Incubation
-
Cells are incubated with the test compounds for a predetermined period to allow for adequate interaction and receptor activation or inhibition.
-
Detection and Measurement
-
Reporter Gene Readout: After incubation, the functional response is measured using a reporter gene assay. This typically involves the detection of luminescent or fluorescent signals proportional to receptor activity.
-
IC50 Calculation: Data from dose-response curves are analyzed to calculate IC50 values, indicating the concentration at which each compound achieves half-maximal inhibition (for antagonists) or activation (for agonists).
Published Data
This study features a detailed cell-based quantitative high-throughput screening (qHTS) assay optimization and the identification of human CAR agonists, as depicted in Figure 1. This figure illustrates the optimization manner of the cellular-based totally qHTS assay, highlighting key steps such as cell line selection, transfection performance, and assay validation. Additionally, it demonstrates the identity of potential hCAR agonists from a large compound library. The parent underscores the importance of optimizing assay conditions to attain high sensitivity and specificity, facilitating the accurate detection of hCAR modulators.
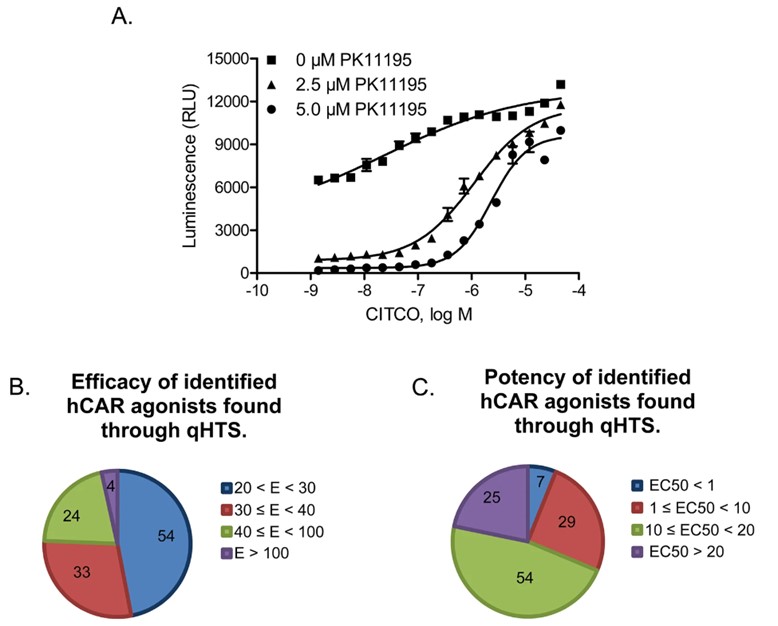 Fig.1 Cell-based qHTS assay optimization and hCAR agonist identification.1
Fig.1 Cell-based qHTS assay optimization and hCAR agonist identification.1
Applications
-
Assessing the function of CAR3 in the regulation of metabolic enzymes.
-
Evaluating the potential for new drugs to interact with other medications metabolized by CAR3-regulated pathways.
-
Screening for toxic metabolites produced via CAR3-regulated metabolic pathways.
-
Identifying compounds that may induce or inhibit CAR3, affecting liver function and overall drug safety.
-
Utilizing CAR3 assay data to predict drug clearance rates and dosage requirements.
Advantages
-
Expertise in the particular characteristics of CAR3, including its constitutive activity and role in liver metabolism.
-
In-depth information on CAR3's regulatory effects on drug-metabolizing enzymes is vital for predicting drug behavior.
-
High Sensitivity and Specificity: Utilization of highly sensitive detection methods to accurately measure CAR3 activity and ligand interactions.
-
Specific assays are tailor-made to detect both agonist and antagonist activities of compounds on CAR3.
At Creative Biolabs, CAR3 nuclear receptor assay stands out as a premier solution for researchers and pharmaceutical developers aiming to advance their drug discovery and development projects. Our expertise, advanced technology, and commitment to quality ensure that you receive precise, reliable, and actionable data. If you are looking to gain deeper insights into the metabolism, safety, and efficacy of your drug candidates through comprehensive CAR3 nuclear receptor assays, feel free to contact Creative Biolabs today.
Reference
-
Lynch, Caitlin, et al. "Quantitative high-throughput identification of drugs as modulators of human constitutive androstane receptor." Scientific reports 5.1 (2015): 10405.
For Research Use Only | Not For Clinical Use



 Fig.1 Cell-based qHTS assay optimization and hCAR agonist identification.1
Fig.1 Cell-based qHTS assay optimization and hCAR agonist identification.1
 Download our brochure
Download our brochure