CreStab™ Technology for Preventing Antibody Aggregation
Therapeutic monoclonal antibodies (mAb) represent the most important protein-based biological drugs. Like all other protein therapeutics, mAb therapeutics may undergo various degradation processes during production, formulation, storage, and transportation. The presence of aggregates is undesirable because they cause loss of activity, reduced solubility, and increased undesirable immunogenicity. Therefore, during the development and production of mAb therapeutics, characterizing mAb aggregates is critical for process development and quality control.
With years of experience in therapeutic antibody development, Creative Biolabs provides a wide range of analytical services to help our customers understand their therapeutic monoclonal antibody candidates in terms of physicochemical properties, biological activity, affinity, and purity. At present, we introduce mAb aggregation prevention as part of the antibody characterization and analysis service portfolio. We have developed a novel technology - CreStab™ to reduce the aggregation of mAb and is expected to improve the stability of human antibodies.
Introduction to CreStab™ Technology
CreStab™ technology optimizes amino acids at specific positions within the complementarity determining region (CDR) of antibodies to obtain fully human antibodies with completely increased stability and reduced tendency to aggregate, which still retains all the characteristics required for therapeutic use. This technology has been validated by improving the stability of many currently available therapeutic antibodies and drug candidates while maintaining binding and other critical functions.
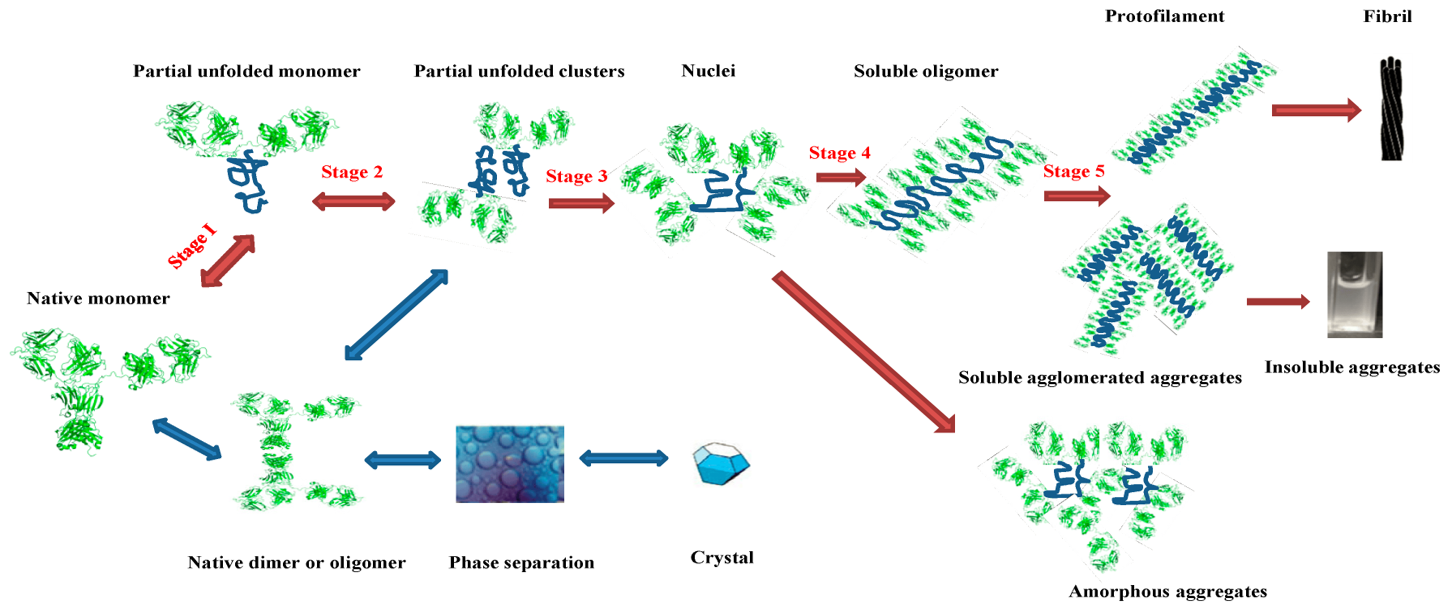 Fig.1 Schematic representation of the protein aggregation process and the possible involved intermediates.1
Fig.1 Schematic representation of the protein aggregation process and the possible involved intermediates.1
Applications of CreStab™ Technology
-
CreStab™ can be used in all antibodies being developed to improve properties and reduce future production and commercial risks.
-
CreStab™ can also improve antibodies that would otherwise fail to meet manufacturability requirements.
-
CreStab™ can be directly applied to a variety of antibody formats, including fragments, bispecific antibodies, and full-length IgG monoclonal antibodies.
-
CreStab™ can be quickly implemented and tested using highly predictive methods and can be applied to phage display libraries.
For further details, please don't hesitate to contact us.
Reference
-
Li, Wei, et al. "Antibody aggregation: insights from sequence and structure." Antibodies 5.3 (2016): 19.
For Research Use Only | Not For Clinical Use


 Fig.1 Schematic representation of the protein aggregation process and the possible involved intermediates.1
Fig.1 Schematic representation of the protein aggregation process and the possible involved intermediates.1
 Download our brochure
Download our brochure