GVHD Immunosuppressive Drug Development Services
Creative Biolabs is at the forefront of CRO companies worldwide in the field of GVHD therapy and immunosuppressive research, offering comprehensive services for GVHD diagnosis, prediction, and drug and therapy development. With state-of-the-art R&D and kg laboratories as well as a commercial-scale production workshop, Creative Biolabs can expedite the development and testing process of GVHD immunosuppressive agents while reducing R&D costs. Our team of GVHD immunosuppressant developers will customize solutions to meet your current and future needs, ensuring efficient and precise technical support.
Introduction
Immunosuppressants are medications that can inhibit abnormal immune reactions in the body, playing a crucial role in preventing and treating organ transplant rejection as well as autoimmune diseases. Specifically, they are essential for preventing graft-versus-host disease (GVHD) by restricting T cell function and inhibiting immune responses at various stages of T cell proliferation, differentiation, and activation. With the increasing incidence of GVHD, there is a growing demand for immunosuppressants. Therefore, the search for new immunosuppressants with high efficiency and low toxicity remains a key focus in GVHD drug development.
Creative Biolabs specializes in transplantation and provides professional solutions for GVHD diagnosis, prevention, and treatment throughout the entire transplantation process. Our goal is to assist customers with GVHD immunosuppressive screening and development. Nowadays, we provide structural properties, pharmacological activities, biosynthetic mechanism analysis, and chemical synthesis services of GVHD-related immunosuppressant molecules, and strive to find potential immunosuppressive analogs from natural products, combined with immunosuppressive activity screening and safety evaluation, to find immunosuppressants with clinical application value.
Common combinations of GVHD immunosuppressants include:
1. Anti-thymocyte globulin (ATG): ATG is primarily used to remove T cells from grafts in haplotype transplantation, non-blood donor transplantation, and partial HLA-matched sibling donor transplantation to significantly reduce the incidence of GVHD.
2. Post-transplant cyclophosphamide (PT/CY): PT/CY prophylaxis alone has been shown to reduce chronic GVHD incidence to 7% among HLA-compatible transplant patients without routine MTX/CNI prophylaxis.
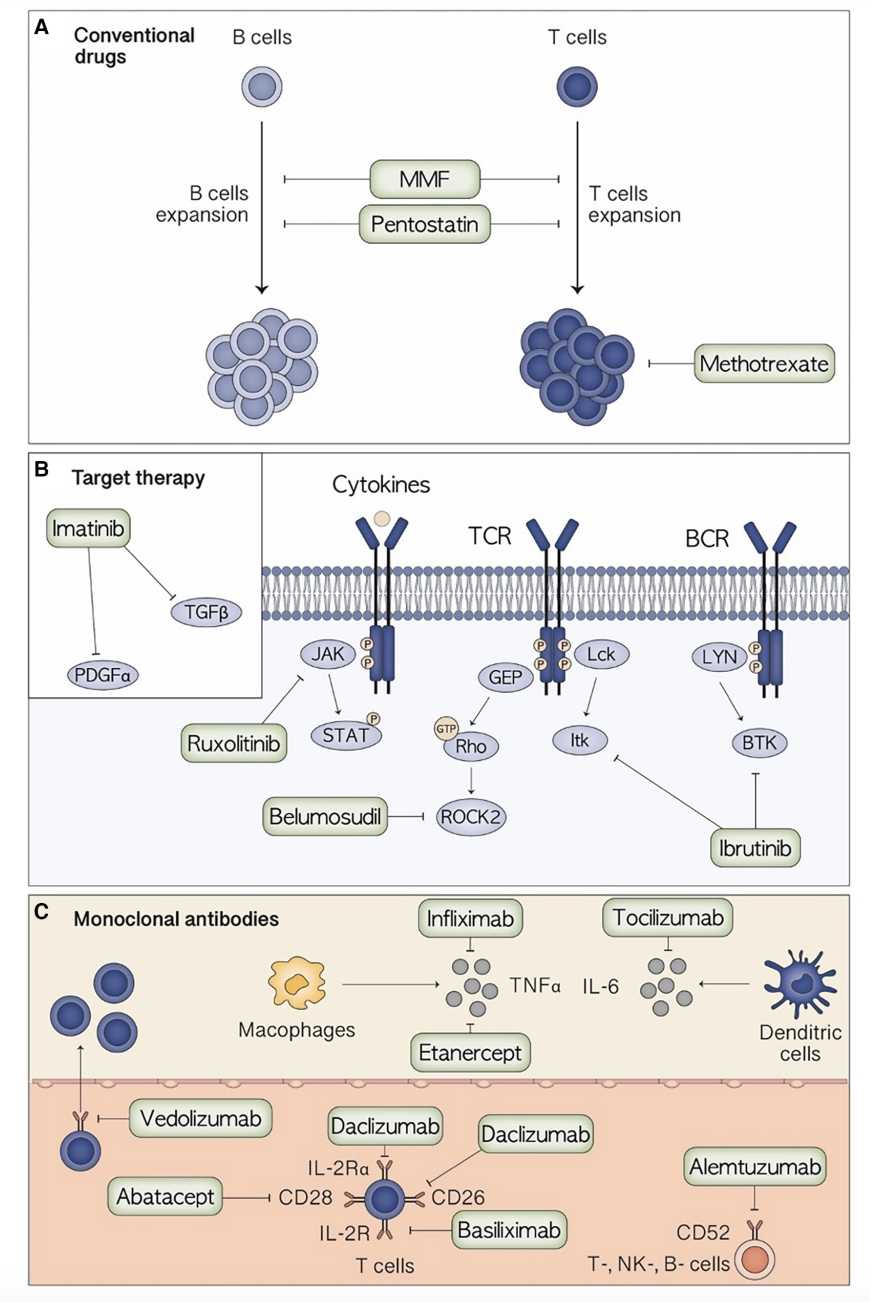 Fig.1 The Main Therapeutic Agents For Acute GVHD Therapy.1
Fig.1 The Main Therapeutic Agents For Acute GVHD Therapy.1
GVHD Immunosuppressant Detection Solutions
Creative Biolabs focuses on scientific research and technology-driven innovation aimed at discovering cutting-edge therapies for GVHD along with novel immunosuppressants that offer breakthrough efficacy. We aim to understand the disease mechanism of GVHD while striving to extend patient survival periods and improve their quality of life through relentless efforts. Moreover, we also provide a range of immunosuppressant detection services such as free drug concentration testing using blood samples or saliva detection methods aimed at promoting accurate, convenient, low-risk detection.
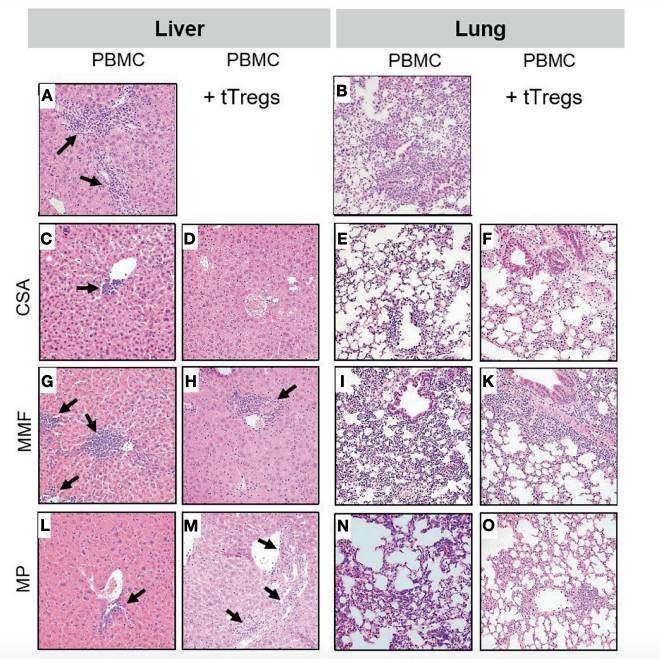 Fig.2 Histopathological Comparison of Liver And Lung Treated With PBMC or GVHD Immunosuppressants.2
Fig.2 Histopathological Comparison of Liver And Lung Treated With PBMC or GVHD Immunosuppressants.2
1. GVHD immunosuppressant detection system based on whole blood/plasma
(1) Detection of total immunosuppressant concentrations in whole blood/plasma.
Currently, our labs primarily conduct tests for the overall drug concentration in whole blood/plasma to assess immunosuppression. Different sample types are selected for drug concentration detection based on the varying distribution of drugs within the body. Our LC-MS/MS detection systems offer high specificity, quantitative accuracy, and traceability to SI units.
(2) Detection of free immunosuppressant concentration in plasma.
Free drug concentration refers to the unbound portion of a drug that is not bound to proteins, which is considered pharmacologically active. Ultrafiltration is typically employed to separate unbound drugs from bound drugs due to its simplicity and higher sample throughput compared to other techniques such as ultracentrifugation and equilibrium dialysis.
(3) Detection of intracellular immunosuppressive concentration in plasma.
Directly assessing the distribution of immunosuppressive agents acting on lymphocytes optimizes their detection according to pharmacological principles. Peripheral blood mononuclear cell (PBMC) determination is usually chosen by our scientists for testing as it more accurately reflects therapeutic target levels and effectively optimizes GVHD treatment strategies.
2. GVHD immunosuppressant detection based on micro-samples
(1) Blood spot drug concentration detection.
The technique of using thousands of blood spots for microsampling has been well-established. By combining paper plate drying matrix with automated dry blood spot analysis, we have overcome interference caused by hematocrit (Hct) effects. Additionally, our LC-MS/MS-based method for detecting multiple immunosuppressants in macules has been established with results consistent with those from venous blood samples.
(2) Volume absorption microsampling drug concentration detection.
Volumetric absorption microsampling (VAMS) represents a new microsampling technology offering fast sampling times, minimal invasiveness, and convenient operation. We utilized an LC-MS/MS method to detect 5 immunosuppressants based on VAMS and fully verified their performance against established standards.
(3) GVHD immunosuppressant detection based on saliva samples
The lipophilicity of immunosuppressants enables free drugs within the human body to effectively penetrate saliva, allowing for noninvasive testing methods utilizing this bodily fluid. Our laboratory has developed an LC-MS/MS-based method capable of simultaneously detecting 5 kinds of immunosuppressants in saliva. Saliva-based immunosuppressant testing is noninvasive, convenient, and patient-friendly.
Drawing on our highly skilled professional technical team, Creative Biolabs delivers personalized GVHD immunosuppressive development and testing services to help accelerate customers' GVHD drug development processes. Your GVHD project deserves the reliability and peace of mind that only comes from partnering with the most trusted and experienced professionals. We are committed to providing high-quality experimental protocols and results for formulating integrated preclinical research protocols for GVHD. Please contact us for timely consultation and collaboration.
References
-
Gottardi, Francesca, et al. "Treatment of steroid-refractory graft versus host disease in children." Frontiers in Transplantation 2 (2023): 1251112.
-
Landwehr-Kenzel, Sybille, et al. "Cyclosporine a but not corticosteroids support the efficacy of ex vivo expanded, adoptively transferred human tregs in gvhd." Frontiers in Immunology 12 (2021): 716629.
For Research Use Only | Not For Clinical Use


 Fig.1 The Main Therapeutic Agents For Acute GVHD Therapy.1
Fig.1 The Main Therapeutic Agents For Acute GVHD Therapy.1
 Fig.2 Histopathological Comparison of Liver And Lung Treated With PBMC or GVHD Immunosuppressants.2
Fig.2 Histopathological Comparison of Liver And Lung Treated With PBMC or GVHD Immunosuppressants.2
 Download our brochure
Download our brochure