GVHD Prediction System based on Gene-Level Markers
The diagnosis and prognosis assessment of GVHD are almost entirely dependent on the clinical symptoms of patients. Creative Biolabs has been working on the mining of ideal gene-level biological markers of GVHD, hoping to objectively evaluate and verify the pathogenesis, physiological state, and pharmacological reaction process of patients with GVHD. They can also predict the risk, prognosis, and treatment response of GVHD after allo-HSCT. Through blood, urine, biopsy tissue, and other samples, we can reveal quantitative changes in the genetic level of patients with GVHD status and screen for potential markers or combinations of markers associated with GVHD.
Introduction
In recent years, numerous studies have identified GVHD-associated genes predominantly situated within protein-coding regions linked to inflammation or immune regulation. For example, tumor necrosis factor (TNF) serves as a crucial inflammatory cytokine encompassing two subtypes: TNF-α and TNF-β, whose correlation with aGVHD incidence and severity has been consistently validated across multiple research. NOD2 acts as an intracellular receptor participating in innate immune responses by binding to bacterial cell wall components, thereby amplifying NFκB activity. Consequently, studies have established that NOD2 variants present in both donors and recipients correlate with heightened risk for GVHD development. Furthermore, polymorphisms within the IFNG gene are implicated in GVHD onset. Specifically, allele IFNG2 exhibits enhanced production capacity for IFN-γ compared to other genotypes leading to augmented secretion of indoleamine 2,3-dioxygenase (IDO) by host antigen-presenting cells (APC), ultimately fostering robust immunotolerance while mitigating GVHD occurrences. Additionally, advancements in epigenetic research have increasingly underscored microRNAs' pivotal roles in GVHD prediction. Alterations observed in specific miRNA levels within serum hold promise as potential predictive markers for aGVHD.
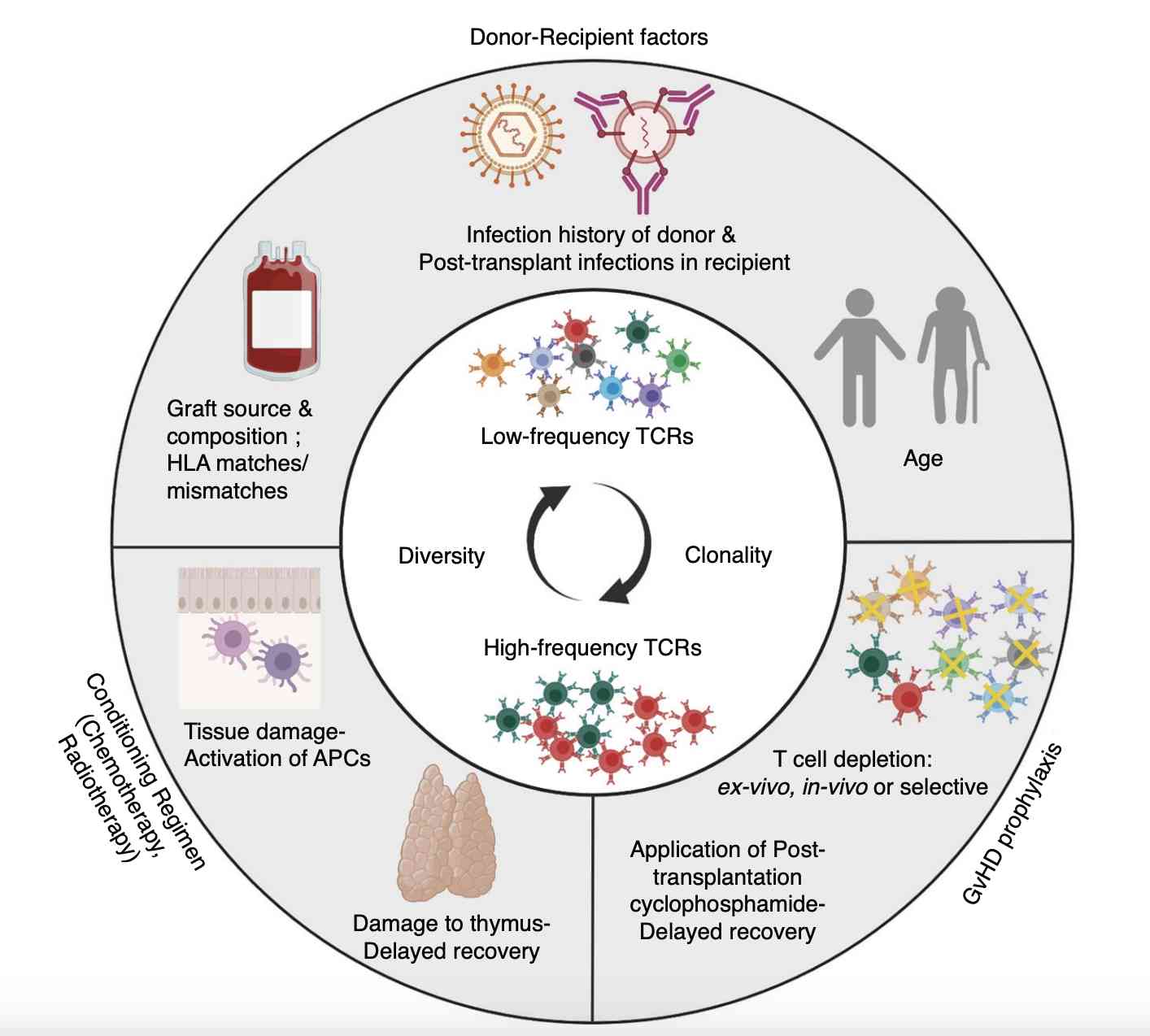 Fig.1 Factors Involved in The Pathophysiology of Graft-versus-host disease (GVHD).1
Fig.1 Factors Involved in The Pathophysiology of Graft-versus-host disease (GVHD).1
Accurate biomarkers are crucial for enhancing GVHD screening, diagnosis, and monitoring. Identifying GVHD biomarkers at the genetic level and developing their clinical applications pose significant challenges. Creative Biolabs has extensive expertise in developing, validating, and analyzing biomarker methods for GVHD, having completed over a dozen confirmations or validations of predictive marker methodologies based on gene expression. Meanwhile, we have an advanced and comprehensive GVHD bioassay platform that encompasses the detection of small and large molecular proteins as well as cellular and genetic-level biomarkers. Till now, we have successfully surmounted these challenges to provide a robust detection tool that enhances patient screening and prognosis for those with GVHD. Through collaboration with us, your journey in cancer biomarker discovery can be facilitated, resulting in meaningful outcomes.
miRNA Biomarker Solutions
Creative Biolabs offers comprehensive solutions for GVHD miRNA, gene expression, transcriptome, and functional studies. Here, you can gain insight into every step of our workflow, facilitating the retrieval of information and products you require. We provide proven solutions for sample processing to RNA/DNA sequencing and expression profiling to enhance your efficiency and expedite GVHD gene biomarker research.
Previous studies have shown that miRNAs have great potential as markers of diagnosis, prognosis, and treatment. MiRNAs in the blood can be used as biological markers for specific diseases such as GVHD. In our labs, we use miRNA expression profiling as one of the primary tools for the discovery of potential biomarkers for GVHD. Our staff combines innovative miRNA-SEQ technology with decades of technical expertise in a variety of GVHD sample areas to provide a one-stop miRNA Sequencing Service.
In addition, Creative Biolabs has developed a unique miRNA detection chip that can specifically detect mature miRNAs and distinguish highly homologous miRNA molecules. The platform also has the characteristics of high specificity and sensitivity. It can be widely used to detect and compare the miRNA expression status of various GVHD samples and to further analyze its regulatory mechanism or biological function by analyzing its regulated target genes.
Case Study
In a recent project, Creative Biolabs has investigated the role of animal and human miRNAs in the pathogenesis of GVHD and has screened a variety of biomarkers and therapeutic targets. For example, we analyzed the differential expression of miRNAs in serum after allo-HSCT and found dozens of miRNAs, including but not limited to miR-146a, miR-323-b, miR-34c, miR-363, miR-4245, miR-29a, miR-181a, miR-3168, miR-662, miR-550a, miR-4295, miR-582, miR-193a, miR-3940, miR-3674, miR-4258 and miR-3678.
Why Choose Us?
-
End-to-end services: Creative Biolabs will take care of every step, from sample preparation to data analysis;
-
Efficient library preparation: removal of splitter dimer and useless RNA by magnetic bead-based method;
-
Elimination of PCR duplication and sequencing bias: Molecular barcodes (UMI) allow quantification of individual miRNA molecules;
-
High-quality sequencing data guarantee: Rich experience in database construction and sequencing, can choose the optimal library construction strategy according to different project requirements, coupled with Creative Biolabs' strict experimental quality control standards so that high-quality sequencing data can be guaranteed;
-
Comprehensive transcriptome analysis: Creative Biolabs performs gene expression quantitative analysis, enrichment analysis, cluster analysis, variable splicing analysis, gene fusion, target gene prediction, targeted interaction analysis, ceRNA, WGCNA, and GSEA analysis according to project type;
-
Publishable data: Creative Biolabs can deliver comprehensive reports and data packages and guide the next steps.
Identifying a candidate biomarker is an essential step in the prediction, diagnosis, and treatment of GVHD. Creative Biolabs combines cutting-edge technology and R&D experience to form a professional GVHD genetic biomarker service team that works efficiently and provides reliable reagents and equipment to ensure you achieve highly sensitive measurements of analytes. If you are interested in our services, please feel free to contact us.
Reference
-
Goel, Manisha, et al. "Potential of TCR sequencing in graft-versus-host disease." Bone marrow transplantation 58.3 (2023): 239-246.
For Research Use Only | Not For Clinical Use


 Fig.1 Factors Involved in The Pathophysiology of Graft-versus-host disease (GVHD).1
Fig.1 Factors Involved in The Pathophysiology of Graft-versus-host disease (GVHD).1
 Download our brochure
Download our brochure