HK-2-based Cytotoxicity Safety Screening Service
Uniting ADME Insights: Elevate Safety with Creative Biolabs' HK-2-Based Cytotoxicity Screening
Cytotoxicity screening is crucial in absorption, distribution, metabolism, and excretion (ADME) studies, integral to predicting compound safety and efficacy in pharmaceutical development. It assesses potential adverse effects on cells, guiding formulation and dosage strategies to enhance therapeutic outcomes.
-
The HK-2 cell line offers several advantages for cytotoxicity screening:
-
Renal Toxicity Modeling: Mimics human kidney tissue, pivotal for evaluating nephrotoxicity risks in drug development.
-
Robust and Reproducible: Provides consistent performance, ensuring reliable toxicity assessment across studies.
-
Early Detection: High sensitivity to detect subtle cytotoxic effects early in the development process, enhancing safety profiling.
-
Versatile Application: Suitable for diverse industries including pharmaceuticals, biotechnology, and chemicals.
Creative Biolabs is excited to announce the launch of our advanced HK-2-based cytotoxicity safety screening service. This service represents our commitment to delivering high-quality solutions in the critical fields of pharmaceutical and biotechnological research. This service demonstrates our commitment to excellence and precision in this critical area of pharmaceutical and biotechnological research. By leveraging our expertise in HK-2-based cytotoxicity screening, Creative Biolabs ensures a thorough evaluation of potential drug candidates and compounds, contributing to the advancement of safer and more effective therapies in the pharmaceutical and biotechnological industries.
Our method employs luminescence-based detection to assess cell viability, cytotoxicity, and mitochondrial respiration using HK-2 cells. The assay utilizes Beetle luciferin and ATP as substrates, with tamoxifen serving as a control inhibitor, ensuring robust and reliable results in functional cytotoxicity testing.
|
Substrate
|
Assay Type
|
Cell Type
|
Control Inhibitor
|
Detection Method
|
|
Beetle luciferin + ATP
|
Functional
|
HK-2
|
Tamoxifen
|
Luminescence
|
Technical Procedure
-
Cell Culture and Preparation
-
HK-2 Cell Line Maintenance: HK-2 cells are cultured in specialized growth media.
-
Cell Seeding: Cells are seeded into multi-well assay plates at predetermined densities to ensure uniformity across samples and facilitate subsequent assay procedures.
-
Treatment and Incubation
-
Test compounds, along with a control inhibitor tamoxifen for comparative analysis, are added to designated wells of the assay plates.
-
Incubation: The plates are then placed in a cell culture incubator set to physiological conditions for a specified incubation period, allowing the compounds to interact with the cells and induce potential cytotoxic effects.
-
Assay Execution
Following the incubation period, a luminescence-based assay is performed to evaluate multiple parameters:
-
Cell Viability: Assessing the overall health and survival of HK-2 cells.
-
Cytotoxicity: Measuring the extent of cell damage or death induced by the tested compounds.
-
Mitochondrial Respiration: Evaluating the metabolic activity of mitochondria within the HK-2 cells, providing insights into cellular function and potential toxic effects on cellular energy production.
-
Substrate Addition: Beetle luciferin and ATP are added to each well to initiate the luminescence reaction, which is indicative of cellular ATP levels and hence metabolic activity.
-
Measurement and Data Collection
-
Luminescence Detection: The luminescent signal is quantified. This step provides quantitative data on cellular viability, cytotoxicity, and mitochondrial function across the tested conditions.
-
Data Acquisition: Real-time or endpoint measurements are taken, capturing detailed kinetic profiles or final luminescence values as per experimental requirements.
-
Data Analysis and Interpretation
-
Data Processing: Raw luminescence data is processed to calculate percentage viability relative to control conditions.
-
Statistical Analysis: Statistical methods are applied to assess the significance of observed changes.
Published Data
This study takes a look at depicts the evaluation of tenofovir's cytotoxic outcomes on HK-2 cells using Trypan Blue Exclusion. The findings reveal a dose-established cut price in mobile viability upon publicity to tenofovir, underscoring HK-2 cells' suitability as a version for assessing nephrotoxicity delivered via using this drug. This model offers precious insights into the mobile mechanisms underlying tenofovir-introduced kidney harm, contributing to the development of more secure recovery techniques.
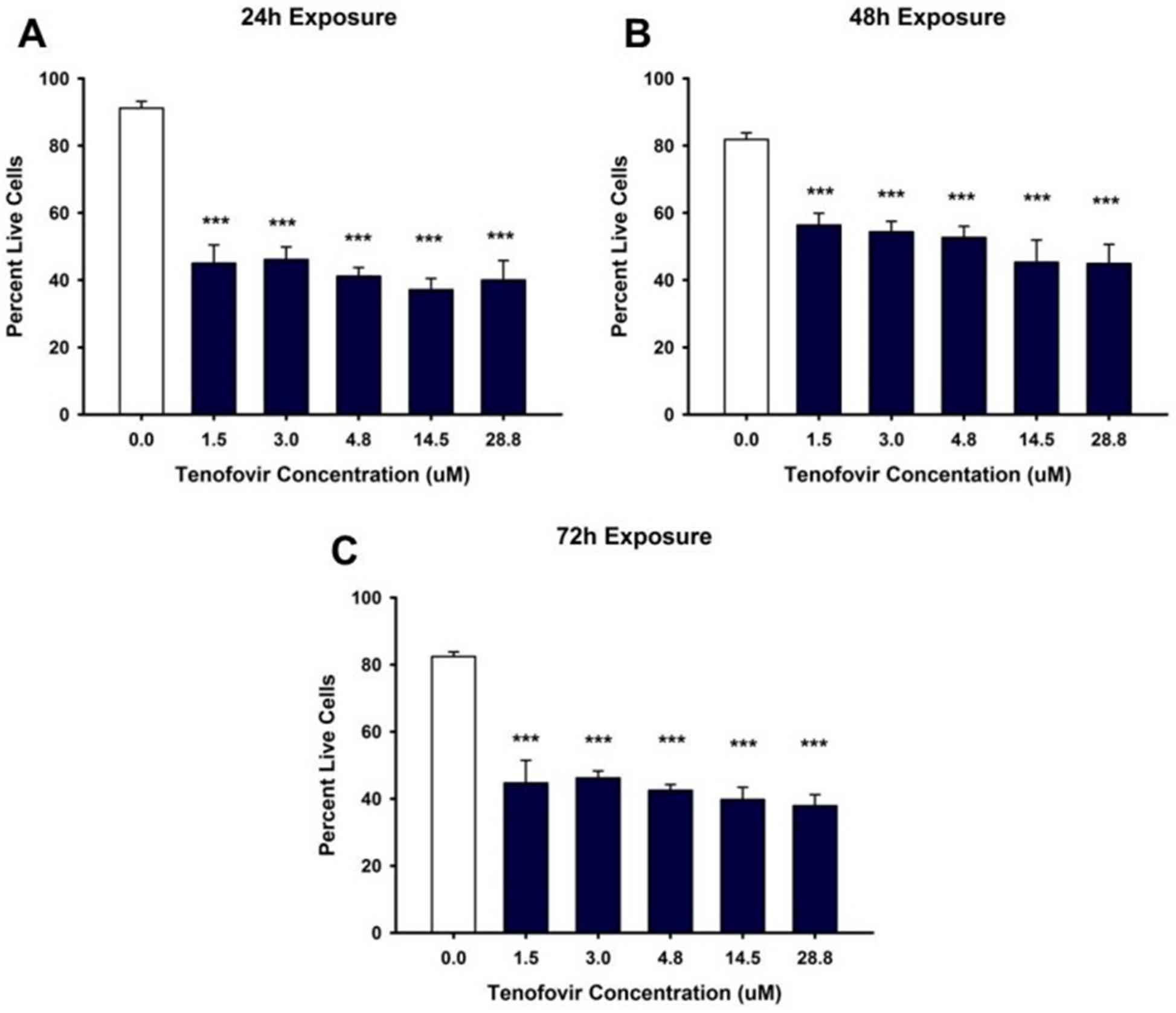 Fig.1 Tenofovir effects on ATP levels in HK-2 cells.1
Fig.1 Tenofovir effects on ATP levels in HK-2 cells.1
Applications
-
Assessing renal protection and toxicity profiles of drug applicants, integrating ADME concerns.
-
Evaluating cytotoxicity of novel compounds and biologics, making sure of product safety and efficacy.
-
Rigorous toxicity screening to ensure customer product safety, leveraging HK-2's predictive abilities.
Advantages
-
Tailored screening protocols align with ADME requirements, optimizing safety evaluation.
-
Supported via skilled scientists talented in HK-2-primarily based cytotoxicity evaluation, making sure thorough analysis.
-
Streamlined strategies for speedy turnaround without compromising accuracy.
-
Innovating drug discovery through superior HK-2 methodologies, selling more secure healing improvements.
By integrating HK-2-based cytotoxicity screening with ADME insights, Creative Biolabs empowers researchers to decorate safety requirements and accelerate drug improvement with self-perception. Contact us to leverage the HK-2 era and develop your innovations in pharmaceuticals and biotechnology, ensuring safer and more powerful recuperation solutions.
Reference
-
Murphy, Rachel A., et al. "Establishment of HK-2 cells as a relevant model to study tenofovir-induced cytotoxicity." International Journal of Molecular Sciences 18.3 (2017): 531.
For Research Use Only | Not For Clinical Use



 Fig.1 Tenofovir effects on ATP levels in HK-2 cells.1
Fig.1 Tenofovir effects on ATP levels in HK-2 cells.1
 Download our brochure
Download our brochure