HUVEC-based Cytotoxicity Safety Screening Service
HUVEC Cell Line Used in Cytotoxicity Safety Screening
Human umbilical vein endothelial cells (HUVECs), derived from the endothelium of veins in the umbilical cord, serve as an exemplary model for studying vascular and endothelial cell behavior. These cells are widely utilized in vitro for cytotoxicity testing due to their well-characterized nature and physiological relevance. In the context of cytotoxicity screening, HUVECs offer critical insights into mechanisms of endothelial damage, inflammatory responses, and cellular viability. This makes them invaluable for assessing the safety profile of compounds before they advance to clinical stages. By using HUVECs, researchers can better predict how a drug or substance may impact vascular health, ultimately contributing to safer therapeutic developments.
HUVEC-based Cytotoxicity Screening Service
At Creative Biolabs, we are committed to delivering comprehensive HUVEC-based cytotoxicity screening services that enable our clients to assess the cytotoxic effects of their compounds efficiently and accurately. By utilizing HUVEC cells in our assays, we provide detailed insights into how potential pharmaceuticals or chemicals affect endothelial cell viability and health. One of the key assays employed in our service is the cell viability assay, with a focus on assessing the host cell ATP content as an indicator of cellular health and viability. This method involves using a luminescent readout, which allows for an accurate and sensitive detection of ATP levels within HUVECs.
-
Luminescent Cell Viability Screening Assay
The cell viability screening assay involves treating cultured HUVEC cells with test compounds followed by incubation. Post-treatment, the ATP content in the cells is assessed using a luminescent readout system. Luminescence is directly proportional to the amount of ATP present, thereby providing a quantitative measure of cell viability. This method not only assures high sensitivity and specificity but also delivers rapid and reproducible results.
-
High-Content Screening Assay
Creative Biolabs also performs cytotoxicity assay utilizing high-content screening (HCS) which integrates automated imaging and quantitative data analysis to measure multiple cellular parameters simultaneously, providing a robust and comprehensive assessment of cytotoxic effects. By leveraging advanced imaging technologies and sophisticated software, we offer detailed insights into cellular morphology, viability, proliferation, and apoptosis. This high-throughput, multi-parametric analysis enables the identification of subtle cytotoxic effects that might be missed by conventional assays, thereby ensuring more accurate and reliable data for informed decision-making in the research and development pipeline.
Details of Our Service
Minimum volume for liquid samples: ~300uL (10 mM).
Dry compound samples: minimum 1 mg.
Samples should be accompanied by comprehensive datasheets including solubility and stability information. Please contact us for more details
Typically, a concentration range depends on the experimental setup.
Typically, our HUVEC-based cytotoxicity screening service will be completed within 4-6 weeks from the receipt of samples.
Clients will receive a comprehensive report including raw data, normalized results, and a thorough interpretation of the findings.
Published Data
Researchers investigated the toxicity of PSNPs and their bioconjugates with HSA and Tf on HUVEC cells. The results indicated that the viability of HUVEC cells remained unaffected by treatment with various concentrations of PSNPs, PSNP-HSA, and Tf bioconjugates.
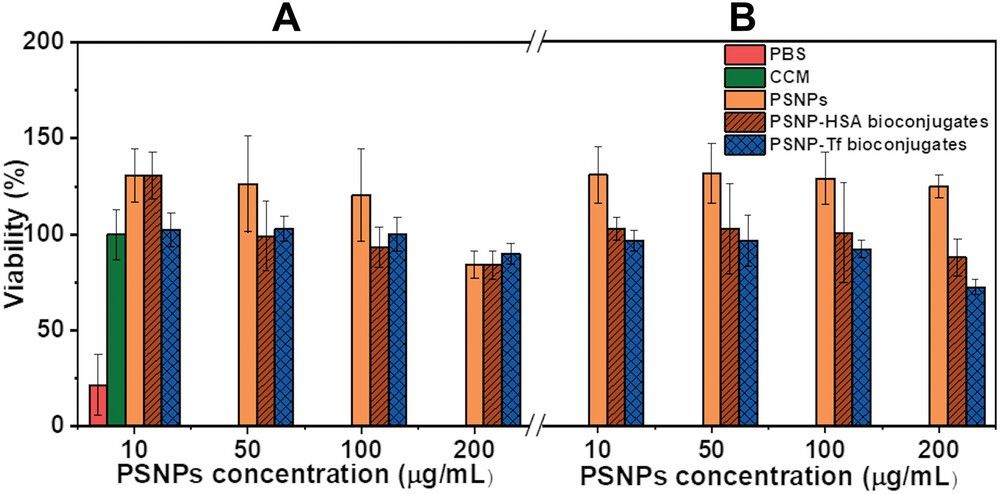 Fig.1 Cytotoxicity assay of HUVEC cells for different PSNP concentrations.1,2
Fig.1 Cytotoxicity assay of HUVEC cells for different PSNP concentrations.1,2
References
-
Meesaragandla, Brahmaiah, et al. "Interaction of polystyrene nanoparticles with supported lipid bilayers: impact of nanoparticle size and protein corona." Macromolecular bioscience 23.8 (2023): 2200464.
-
Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only | Not For Clinical Use


 Fig.1 Cytotoxicity assay of HUVEC cells for different PSNP concentrations.1,2
Fig.1 Cytotoxicity assay of HUVEC cells for different PSNP concentrations.1,2
 Download our brochure
Download our brochure