In Vitro Cytochrome P450 2B6 (CYP2B6) Intrinsic Clearance Measurement Service
Reaction phenotyping studies are essential during the early stages of drug discovery and development to evaluate the metabolic pathways of new drug candidates. Creative Biolabs is dedicated to offering a comprehensive range of CYP phenotyping assays to help guide drug development, optimize dosing regimens, and ensure the safety and efficacy of pharmaceutical compounds.
CYP2B6 Intrinsic Clearance Measurement Service
Intrinsic clearance is a measure of the enzyme's ability to metabolize a specific substrate. The assay typically involves incubating a known concentration of a CYP2B6 substrate with liver microsomes or recombinant enzymes that express CYP2B6 and measuring the parent compound remaining or metabolite formation over time. By comparing the rate of parent compound deletion or metabolite formation to controls, the intrinsic clearance of CYP2B6 can be calculated.
CYP2B6 intrinsic clearance measurement is a method used to quantify the rate at which a drug is metabolized by the CYP2B6 enzyme. Regulatory agencies often require CYP reaction phenotyping data as part of drug approval submissions to ensure the safety and efficacy of new drugs. Based on rich expertise and experience in pharmaceutical drug development, scientists at Creative Biolabs design and conduct CYP2B6 phenotyping studies to assess the metabolic activities of CYP2B6, understanding the role of CYP2B6 enzymes in drug metabolism and potential drug-drug interactions. There are several methods to measure CYP2B6 intrinsic clearance, including in vitro studies using recombinant enzymes, liver microsomes, or hepatocytes. By utilizing recombinant CYP2B6, our laboratory offers a comprehensive analysis of substrate depletion measurement to evaluate the intrinsic clearance of your test drug. This assay is essential for predicting drug clearance in vivo and for understanding potential pharmacokinetic interactions between drugs that are metabolized by CYP2B6.
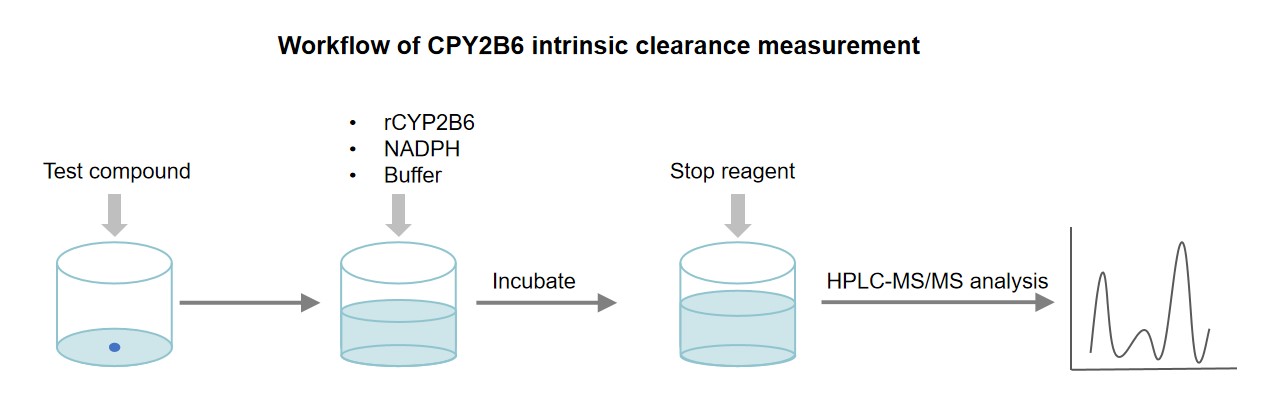
General Procedure
The following is a general procedure for the CYP2B6 intrinsic clearance measurement using recombinant CYP2B6 enzyme:
-
The recombinant CYP2B6 enzyme, NADPH cofactor, test compound, and buffer incubate at a controlled temperature (usually 37°C) for a specified period.
It is essential to ensure that the concentration of the enzyme, NADPH, and test compound are optimized for the assay.
-
An appropriate quenching agent or solvent terminates the reaction. Ensure that the quenching agent does not interfere with the analysis of the metabolites.
-
The remaining parent compound is analyzed by HPLC-MS/MS.
-
The intrinsic clearance of CYP2B6 for the test compound is determined by the rate of test compound deletion over time.
Highlights
-
Comprehensive and tailored solutions to meet your research needs
-
Validate the assay using appropriate controls
-
Careful data interpretation and validation
For Research Use Only | Not For Clinical Use



 Download our brochure
Download our brochure