MSC-Based Immunomodulation GVHD Solutions
Due to its capacity to differentiate into various cell types and its immunomodulatory function, MSC has become a prominent target for GVHD therapy. Creative Biolabs recognizes the advantages of utilizing MSC in GVHD therapy and offers comprehensive solutions to support your development of MSC-based GVHD therapy at all stages. Our objective is to assist you throughout your research by providing high-quality materials and services.
Introduction
Mesenchymal stem cells (MSCs) are highly differentiated and self-renewing pluripotent, can differentiate into several other mesenchymal tissues, such as muscle cells and fat cells, and can also differentiate into different lineages in vitro, including epithelial cells, neuronal cells, astrocyte morphology, endothelial cells, and smooth muscle cells.
Recent studies have shown that bone marrow MSC in patients with immune-related blood system diseases has abnormal growth or function. Based on the immunomodulatory function of MSC and its regulatory effect on hematopoiesis, allo-HSCT combined infusion of MSC from healthy donors may cure such diseases. It has great clinical potential in preventing or treating GVHD after allo-HSCT and promoting HSC implantation. Moreover, the soluble substances secreted by MSC, including exosomes and microvesicles, can be used as therapeutic agents as a new cell-free alternative tool because they are rich in therapeutic miRNA, mRNA, cytokines, and other components.
Creative Biolabs regulates the isolation, culture, and amplification of MSCs to help customers determine optimal cell numbers, timing, and safety to construct, optimize, and validate the immunomodulatory effects of MSCs in GVHD models, and to actively advance MSC therapy as a promising therapeutic strategy for GVHD. Currently, we mainly provide three MSCs mechanism analysis services for GVHD therapy discovery.
-
Analysis of interaction between MSC and innate immune cells and adaptive immune cells;
-
Analysis of immunomodulatory effects of a series of cytokines, chemokines, and soluble receptors secreted by MSC;
-
Analysis of the immunomodulatory role of extracellular vesicles (EVs) in intercellular transfer based on the release of functional proteins and lipids by MSC.
In addition, we provide analyses of factors associated with MSC treatment in GVHD, including, but not limited to, soluble factors, oxygen concentration, Toll-like receptor ligand (TLR), MSC injection dose, immunosuppressive use, and temperature control.
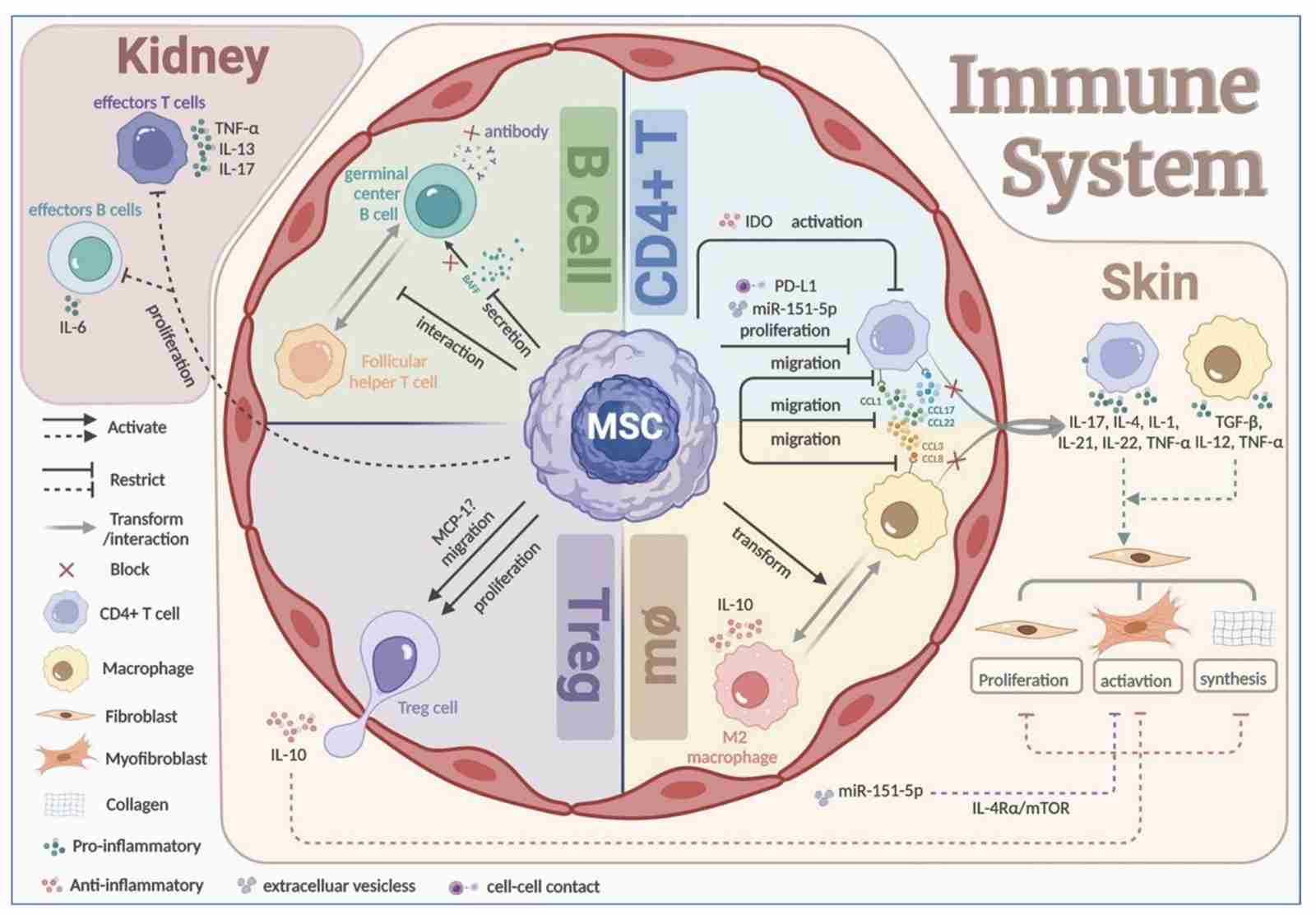 Fig.1 The Therapeutic Role of MSCs to The GVHD.1
Fig.1 The Therapeutic Role of MSCs to The GVHD.1
GVHD Immunosuppressant Detection Solutions
In the past decade, several new therapeutic approaches, including innovative immunomodulatory drugs, physical therapy, and cell therapy, have been gradually employed in the prevention and treatment of GVHD. The potential for these approaches is promising. Among them, cell therapy has emerged as a popular focus of research in recent years. MSCs are easily obtainable, abundant in sources, and possess immunomodulatory capabilities and low immunogenicity. They have been extensively investigated for their potential to prevent, diagnose and treat GVHD. We will provide an analysis of the impact and mechanism of MSC inhibition on GVHD as well as assess the risks associated with GVL effects and infection complications to enhance the therapeutic efficacy of allo-HSCT.
The definition of MSC is still controversial, and the research on its mechanism of action and clinical effects is still being explored. Therefore, we provide evaluation and validation services for the development of MSC-based GVHD therapies. We will provide a complete assessment of molecular markers and differentiation potential, tumorigenicity, identification and titer, immunomodulatory activity, in vivo distribution and homing ability, purity, and impurity.
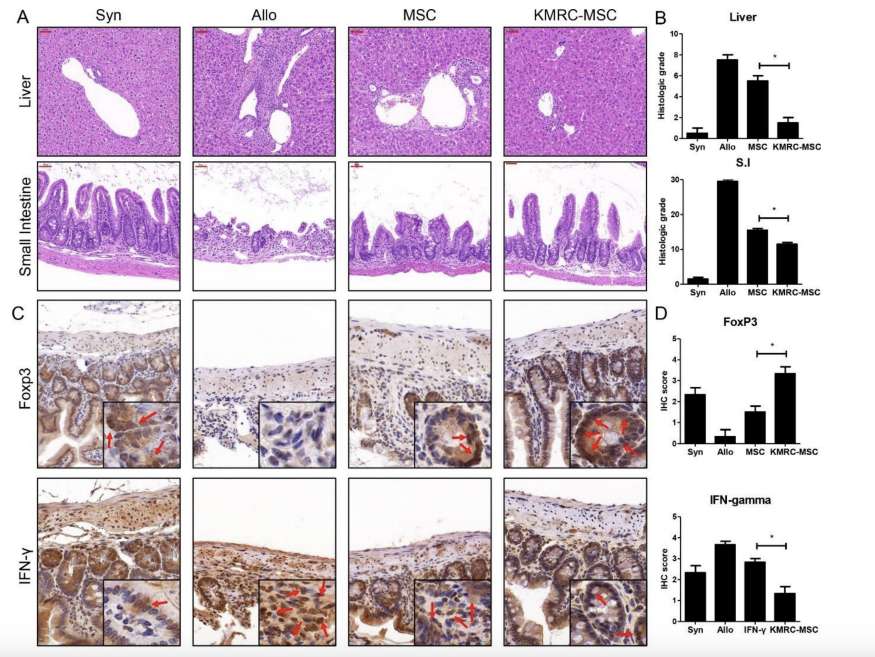 Fig.2 MSCs Attenuate The GVHD Response.2
Fig.2 MSCs Attenuate The GVHD Response.2
In general, we segregate stage-specific embryonic antigen-4 (SSEA-4), CD146, and stromal precursor antigen-1 (Stro-1). Stro-1 can be used as a marker of MSCs to detect the expression level of MSC molecular markers from different tissue sources. We have developed a series of risk analysis assays for tumor formation, Including karyotype analysis, teratoma formation tests, soft AGAR cloning tests, genomic hybridization, in-situ hybridization (FISH), and polymerase chain reaction (PCR), to continuously monitor the risk of ectopic tissue formation. In our labs, we will combine different methods, such as ribonucleic acid quantitative analysis, marker flow detection, secreted protein analysis, and immune cell response analysis, to characterize the immune function of MSC cells, and provide data support for the determination of biological efficacy of MSC in GVHD therapy.
Furthermore, we have recently revealed its immunomodulatory role by establishing an MSC-immune cell interaction analysis system. Meanwhile, we use IFN-gamma and tumor necrosis factor-α (TNF-α) incubated cells to detect the immunoregulatory capacity of MSC and purify immune cells to analyze the immune function of MSC.
-
Controllable MSC variability ensures reproducibility of the experiment;
-
Automated analysis of MSC characterization;
-
High-throughput analysis to accelerate the exploration of new insights into the treatment of GVHD;
-
Advanced phenotypic and functional analysis of MSC and affected tissues;
-
Precise MSC Key Quality Attribute (CQA) determination.
Creative Biolabs is a global leader in the development of GVHD innovative therapies, focusing on the continuous promotion of GVHD, and is committed to creating safe, effective, controllable quality, affordable, and innovative GVHD therapeutic products or therapies to fill the gap in the global market. Please contact us for timely consultation and collaboration.
References
-
Yang, Han, et al. "Mesenchymal stem cell-based therapy for autoimmune-related fibrotic skin diseases-systemic sclerosis and sclerodermatous graft-versus-host disease." Stem Cell Research & Therapy 14.1 (2023): 372.
-
Gil, Sojin, et al. "Mesenchymal stem cells preconditioned with a TLR5 agonist enhanced immunoregulatory effect through M2 macrophage polarization in a murine graft-versus-host disease model." International Journal of Medical Sciences 21.9 (2024): 1649-1660.
For Research Use Only | Not For Clinical Use


 Fig.1 The Therapeutic Role of MSCs to The GVHD.1
Fig.1 The Therapeutic Role of MSCs to The GVHD.1
 Fig.2 MSCs Attenuate The GVHD Response.2
Fig.2 MSCs Attenuate The GVHD Response.2
 Download our brochure
Download our brochure