NIH/3T3-based Cytotoxicity Safety Screening Service
Advancing Safety with NIH/3T3 Precision
Cytotoxicity safety screening is a fundamental step in drug development and biomedical research, aimed at evaluating the toxic effects of new compounds on cultured cells. This screening helps identify potential cytotoxic effects early in the development process, ensuring that only safe and effective compounds progress to later stages of testing and clinical trials. Cytotoxicity assays provide critical data for safety assessments, regulatory submissions, and the optimization of therapeutic agents, ensuring the highest standards of patient safety and treatment efficacy.
Features of NIH/3T3 cells:
-
Origin: Derived from mouse embryonic fibroblasts.
-
Stable Growth: Known for their stable growth characteristics and easy maintenance in culture.
-
Consistency: Provide consistent and reproducible results, essential for reliable cytotoxicity screening.
-
Versatility: Widely used in various types of biomedical research, including cancer, toxicology, and drug development.
-
Relevance: Serve as a model system for studying fibroblast behavior, tissue repair, and fibrosis.
-
Robustness: Exhibit robust responses to a variety of compounds, making them ideal for high-throughput screening.
-
Validation: Their established use in cytotoxicity assays helps validate the safety and efficacy of new therapeutic agents.
At Creative Biolabs, we are proud to introduce our NIH/3T3-based cytotoxicity safety screening service, showcasing our expertise and commitment to delivering exceptional biomedical research solutions. Complementing our In Vitro ADME services, specifically designed to support the development of immunotherapies. With rigorous methodologies and advanced technologies, we ensure precise evaluation of cytotoxic effects to support the development of safe and effective therapeutic agents.
Our assay utilizes Beetle luciferin combined with ATP as the substrate to produce luminescent signals in NIH/3T3 cells. The addition of a control inhibitor ensures the accuracy and reliability of the results, allowing us to assess functional responses effectively.
Assay Information:
|
Substrate
|
Assay Type
|
Cell Type
|
Control Inhibitor
|
Detection Method
|
|
Beetle luciferin + ATP
|
Functional
|
NIH/3T3a
|
Tamoxifen
|
Luminescence
|
Workflow
|
1. Cell Culture Preparation
|
-
NIH/3T3 cells are cultured in growth media optimized for maintaining cell health and viability.
|
|
2. Assay Plate Preparation
|
-
96-well assay plates are carefully prepared to ensure consistent cell seeding and experimental conditions.
|
|
3. Cell Seeding and Incubation
|
-
NIH/3T3 cells are seeded into the assay plates at a specified density and allowed to adhere overnight, forming a uniform cell monolayer.
|
|
4. Compound Treatment
|
-
Test compounds, including Tamoxifen as a control inhibitor, are added to the assay plates at varying concentrations.
|
|
5. Substrate Addition:
|
-
Beetle luciferin + ATP substrate mixture is added to each well to initiate the luminescent reaction.
|
|
6. Incubation and Measurement
|
-
Assay plates are incubated under controlled conditions to allow compounds to interact with cells and substrates.
-
Luminescence, indicative of cell viability and cytotoxicity, is measured using a luminometer.
|
|
7. Data Analysis
|
-
Fluorescence responses are quantified and analyzed to determine cytotoxic effects of test compounds.
-
Dose-response curves are generated, and IC50 values are calculated to assess compound potency and toxicity.
|
Published Data
This study specifically examines the effects of chloronaphtoquinone quercetin 5 on NIH-3T3 cell proliferation and growth inhibition. The data reveals a pronounced reduction in cell proliferation rates and significant growth inhibition upon exposure to chloronaphtoquinone quercetin 5. This highlights the compound's potent cytotoxicity against NIH-3T3 cells, suggesting its potential as a therapeutic agent for targeting aberrant cell growth. Further research into the underlying mechanisms and specific therapeutic applications of chloronaphtoquinone quercetin 5 is warranted to explore its clinical implications in treating conditions involving uncontrolled cell proliferation.
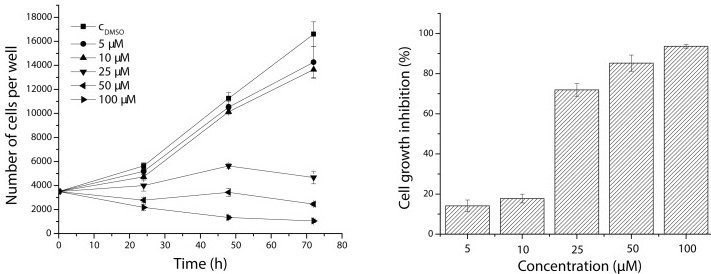 Fig.1 Effect of chloronaphtoquinone quercetin 5 on cell proliferation and growth inhibition of NIH-3T3 cells after 72 h of exposure.1
Fig.1 Effect of chloronaphtoquinone quercetin 5 on cell proliferation and growth inhibition of NIH-3T3 cells after 72 h of exposure.1
Applications
-
Essential for evaluating the safety profiles of pharmaceutical compounds during preclinical development stages, ensuring compounds are safe for further testing and potential clinical trials.
-
Facilitates the assessment of potential toxic effects of drug products, providing valuable insights into their impact on cellular health.
-
Supports research endeavors focused on understanding cytotoxic mechanisms, exploring compound interactions, and optimizing therapeutic efficacy and safety.
Advantages
-
NIH/3T3 cells are nicely installed for strong growth and steady response, making sure dependable and reproducible experimental outcomes.
-
Uses Beetle luciferin + ATP substrate and luminescence detection strategies, enabling touchy and quantitative evaluation of cytotoxicity.
-
Tamoxifen and other management inhibitors were delivered to ensure the validity of the trial and to boost the reliability of the information.
Our detailed technical procedure ensures thorough evaluation of cytotoxicity using NIH/3T3 cells, supporting the rigorous assessment of drug safety and efficacy profiles, which is essential for advancing biomedical research and therapeutic development. Choose Creative Biolabs for your NIH/3T3-based cytotoxicity safety screening needs. Our specialized expertise and commitment to quality ensure rigorous evaluation of cytotoxic effects, empowering your research and development initiatives. Feel free to reach out to us now to discover how our specialized services can support your research objectives.
Reference
-
Danihelová, Martina, et al. "Antioxidant action and cytotoxicity on HeLa and NIH-3T3 cells of new quercetin derivatives." Interdisciplinary toxicology 6.4 (2013): 209-216.
For Research Use Only | Not For Clinical Use


 Fig.1 Effect of chloronaphtoquinone quercetin 5 on cell proliferation and growth inhibition of NIH-3T3 cells after 72 h of exposure.1
Fig.1 Effect of chloronaphtoquinone quercetin 5 on cell proliferation and growth inhibition of NIH-3T3 cells after 72 h of exposure.1
 Download our brochure
Download our brochure