Plasma Stability Determination Service
Plasma stability is a critical component in the Absorption, Distribution, Metabolism, and Excretion (ADME) profiling of new chemical entities during the drug discovery process. It refers to the ability of a compound to remain intact in plasma without undergoing degradation by plasma enzymes such as esterases, amidases, or proteases. Evaluating the stability of potential drugs in plasma provides essential insights into their pharmacokinetics, bioavailability, and overall efficacy. Given its importance, Creative Biolabs offers an advanced plasma stability determination service that ensures high-quality, reliable data to support drug development endeavors.
Applications of Plasma Stability Determination
-
Understanding Pharmacokinetic Properties of Drug Candidates
Plasma stability is indispensable for understanding the pharmacokinetic properties of drug candidates. Compounds that are unstable in plasma are subjected to rapid degradation, which can lead to rapid clearance, short half-life, and ultimately poor in vivo efficacy. For instance, molecules containing ester or amide-linked groups are particularly prone to enzymatic hydrolysis by plasma esterases and amidases. Such instability can result in fast clearance and ambiguous pharmacokinetic (PK) data.
-
Identifying Structural Liabilities
A plasma stability screen can alert researchers to structural liabilities in chemical compounds early in the drug development process. Compounds with functional groups such as esters, amides, lactones, lactams, carbamides, sulphonamides, and peptic mimetics are known to be susceptible to hydrolysis in plasma. By identifying these vulnerable structures, researchers can prioritize and refine compounds for further in vivo studies.
-
Application in Pro-drug Development
For pro-drugs designed to be activated in plasma, the stability determination assays provide valuable kinetic information. These assays help in monitoring the conversion rate of the pro-drug to its active form, thereby assisting in ranking and selecting the best candidates for development.
Plasma Stability Determination Service at Creative Biolabs
Creative Biolabs offers a comprehensive plasma stability determination service as part of its in vitro ADME screening suite. Our service is geared towards providing consistent, high-quality data with cost-efficiency through a highly automated approach. In addition to evaluating plasma stability, our service also extends to metabolite profiling studies supported by high-resolution, accurate mass spectrometry. Our services cover multiple species, including humans, rats, and mice, allowing for cross-species comparisons and the identification of potential interspecies differences in plasma stability.
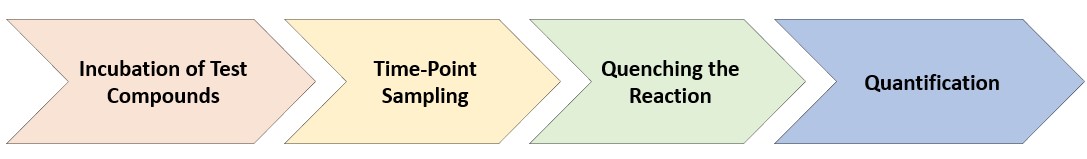 Fig.1 Experimental procedure of our plasma stability determination.
Fig.1 Experimental procedure of our plasma stability determination.
Our plasma stability report includes:
The percentage of the parent compound remaining in plasma over time
Half-life (T½) determinations
Full study report with graphical representations and interpretations
Typically, an accurately weighable quantity of dry compound (~1 mg or 2 µmol) or 50 µL of a 10-20 mM stock DMSO solution suffices.
Features of Our Plasma Stability Determination Service
-
High-Resolution, Accurate Mass Spectrometry
-
Services Covering Multiple Species
-
Comprehensive Reporting
Frequently Asked Questions and Answers
Q1: How accurate are the plasma stability assays conducted at Creative Biolabs?
A1: Our plasma stability assays are highly accurate. We use high-resolution, accurate mass spectrometry for precise metabolite profiling and validate our methods using known control compounds. Recovery tests are also performed to ensure extraction efficiency.
Q2: What species can Creative Biolabs perform plasma stability assays on?
A2: We offer plasma stability assays for a range of species including humans, rats, mice, dogs, and monkeys. Additional species can be accommodated upon request.
Q3: What are the main deliverables from the plasma stability assay service?
A3: The main deliverables include a comprehensive study report detailing the percentage of parent compound remaining, half-life calculations, and detailed analysis of any formed metabolites. The report provides critical data to guide drug development decisions.
Creative Biolabs' Plasma Stability Determination Service offers a robust, scientifically backed approach to evaluating the stability of drug candidates during the early stages of development. For further inquiries and customized service options, please contact us directly.
For Research Use Only | Not For Clinical Use


 Fig.1 Experimental procedure of our plasma stability determination.
Fig.1 Experimental procedure of our plasma stability determination.
 Download our brochure
Download our brochure