Quality Control for In Vivo Assays
Creative Biolabs is committed to building the most sophisticated service platform for in vivo research in cell and gene therapy. We are able to conduct projects in a standardized program that complies with GLP (FDA / OECD) regulations to provide our clients with the most qualified pre-clinical testing services. From the most important project design to the final data interpretation, Creative Biolabs rigorously checks and verifies every step.
General Concepts in Method Development and Validation of an In Vivo Model
Typically, the purpose of method validation is to determine the biological and/or pharmacological activity of CAR / TCR cells. The validation process stems from the identification and/or design of animal models and method development and continues throughout the assay life cycle. During method development, we select assay conditions and procedures to minimize the impact of potentially ineffective sources (such as so-called false positives or false negatives) on the measurement of analytes or biological endpoints (e.g., biochemical, physiological, or behavioral changes).
We perform three basic general areas of method development and validation:
-
Pre-study validation (identification and design phase)
-
In-study validation (development and production phase)
-
Cross-validation or method transfer verification.
In Vivo Assay Design Considerations
Good experimental design is important to answer interesting research questions in an unbiased manner. Our scientists consider the following factors in assay design:
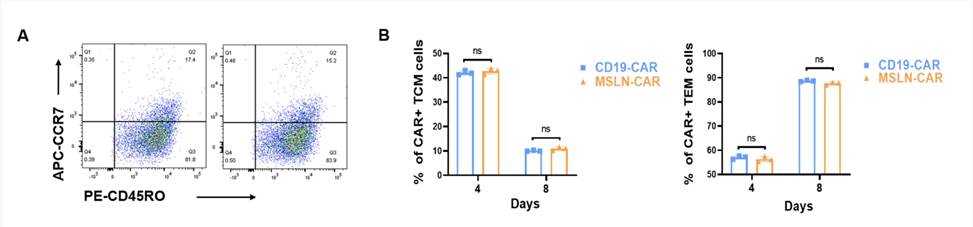 Fig.1 Antitumor efects of MSLN-CAR T cells on ovarian cancer cell xenografts in vivo.1
Fig.1 Antitumor efects of MSLN-CAR T cells on ovarian cancer cell xenografts in vivo.1
-
Timing of measurement, frequency of administration and route
-
Species to use
-
Determining the appropriate sample size
-
Determining the randomization scheme
-
Appropriate random allocation of animals to the treatment group
-
Appropriate selection of dose levels
-
The best choice for the control group
-
The best time to collect samples

Design Strategy
In vivo studies should be designed to make all meaningful biological effects statistically significant. Biologically meaningful effects are not always well known, in which case a range of reasonable effects can be considered. The types of variables that our experimental design takes into account include:
-
Manipulating variables (independent/interpreted variables)
-
Response variable (dependency/result variable)
-
Extraneous variable (uncontrolled / random)
For more details, please feel free to contact us for project quotations and more detailed information.
Reference
-
Guo, Jing, et al. "Mesothelin-based CAR-T cells exhibit potent antitumor activity against ovarian cancer." Journal of Translational Medicine 22.1 (2024): 367.
For Research Use Only | Not For Clinical Use


 Fig.1 Antitumor efects of MSLN-CAR T cells on ovarian cancer cell xenografts in vivo.1
Fig.1 Antitumor efects of MSLN-CAR T cells on ovarian cancer cell xenografts in vivo.1
 Download our brochure
Download our brochure