SH-SY5Y-based Cytotoxicity Safety Screening Service
Ensuring Safety with Precision: SH-SY5Y Cytotoxicity Screening
Cytotoxicity safety screening is a crucial component of the absorption, distribution, metabolism, and excretion (ADME) process, ensuring that compounds are not only pharmacologically effective but also safe for further development. In the stages of development, this evaluation helps detect substances to ensure the safety and effectiveness of new treatments. Through the use of dependable tests, cytotoxicity screening offers data that shape the improvement and enhancement of drug candidates ultimately leading to safer and more efficient therapies.
At Creative Biolabs we stand out in delivering expert cytotoxicity safety screening services using the SH SY5Y neuroblastoma cell line. Our dedication, to accuracy, dependability, and cutting-edge techniques guarantee that our clients receive top-notch results. With our proficiency in neurotoxicity testing, we take a leading role in assisting the creation of efficient neurotherapeutic compounds establishing us as the partner, for your cytotoxicity screening requirements.
Characteristics of SH-SY5Y cells:
-
Human Origin: Derived from human neural crest cells, SH-SY5Y cells closely mimic human neuronal physiology.
-
Neuronal Properties: They exhibit many features of mature neurons, including the ability to form neurites, express neurotransmitter receptors, and perform synaptic functions.
-
Versatility: SH-SY5Y cells can differentiate into various neuronal subtypes under specific conditions, making them useful for studying a wide range of neurological processes and toxicological effects.
-
Reproducibility: These cells provide consistent and reproducible results, which are essential for reliable cytotoxicity assessments.
The use of SH-SY5Y cells in cytotoxicity safety screening is particularly valuable for evaluating the neurotoxic effects of compounds, contributing to the development of safer neurotherapeutic agents and enhancing our understanding of neurodegenerative diseases and neuronal damage mechanisms.
Assay Information:
Our SH-SY5Y-based cytotoxicity safety screening service utilizes Beetle luciferin + ATP as the substrate and tamoxifen as the control inhibitor in a functional assay with SH-SY5Y cells, detected through luminescence.
|
Substrate
|
Assay Type
|
Cell Type
|
Control Inhibitor
|
Detection Method
|
|
Beetle luciferin + ATP
|
Functional
|
SH-SY5Y
|
Tamoxifen
|
Luminescence
|
Workflow
-
Cell Culture Preparation
-
Media Preparation: Grow SH-SY5Y cells in DMEM/F-12 supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin.
-
Plating Cells
-
Cell Harvesting: Detach SH-SY5Y cells using trypsin-EDTA, then count and assess cell viability.
-
Seeding: Seed the cells into a 96-well plate at the optimal density for the assay.
-
Compound Treatment
-
Preparation: Prepare serial dilutions of the test compounds in appropriate solvents.
-
Application: Add the test compounds to the wells in triplicate, including vehicle controls and tamoxifen as the positive control inhibitor.
-
Incubation: Incubate the cells with the compounds for 24-72 hours, depending on the experimental requirements.
-
Substrate Addition
-
Preparation: Prepare the Beetle luciferin + ATP substrate solution.
-
Addition: Add the substrate to each well to initiate the luminescence reaction.
-
Luminescence Detection
-
Measurement: Use a luminescence microplate reader to measure the luminescence signal from each well.
-
Recording: Record the luminescence intensity.
-
Data Analysis
-
Processing: Analyze the luminescence data to evaluate the cytotoxic effects of the test compounds on SH-SY5Y cells.
-
Calculations: Calculate IC50 values and compare them with controls.
Published Data
This research shares the outcomes of tests done on SH SY5Y cells to measure their viability following a 3-hour exposure, to cotinine and nicotine. The findings suggest that both cotinine and nicotine have effects on SH SY5Y cells in a dosed manner. Higher doses of these substances notably decreased cell viability indicating harm to the system. This study highlights the significance of using SH SY5Y cells for assessing neurotoxicity as they offer a model for studying how chemicals impact nerve cells. The results from this research enhance our knowledge of the safety profiles of cotinine and nicotine stressing the importance of cytotoxicity evaluations, in developing treatments.
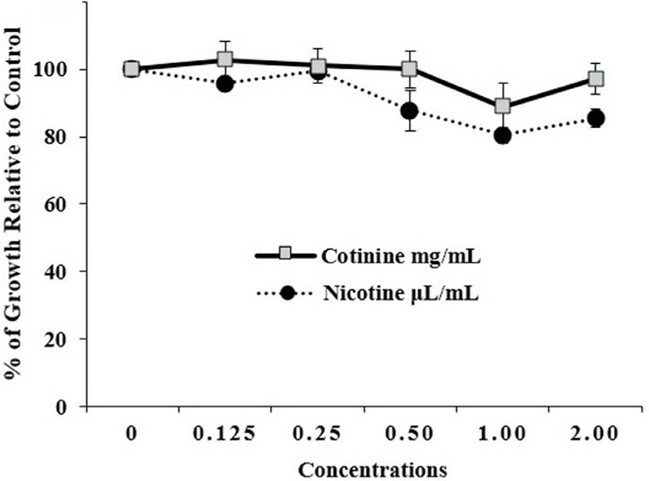 Fig.1 Cell viability evaluation of SH-SY5Y after cotinine and nicotine treatment.1
Fig.1 Cell viability evaluation of SH-SY5Y after cotinine and nicotine treatment.1
Applications
-
Evaluating the potential neurotoxic effects of new drug candidates using SH-SY5Y cells to mimic human neuronal responses accurately.
-
Assisting in the development of neurotherapeutics by identifying compounds that exhibit adverse effects on SH-SY5Y neuronal cells.
-
Utilizing SH-SY5Y cells to study the mechanisms of neuronal cell damage and death in neurodegenerative diseases, aiding in the discovery of potential therapeutic targets and treatments.
-
Providing comprehensive safety profiles for new pharmaceuticals by screening for cytotoxic effects on SH-SY5Y cells.
Advantages
-
SH-SY5Y cells are derived from human neuroblastoma, making them highly relevant for studying human neuronal responses.
-
The ability of SH-SY5Y cells to differentiate into mature neurons allows for more accurate modeling of neurotoxic effects.
-
Using advanced luminescence-based assays, such as Beetle luciferin + ATP substrate with tamoxifen as a control inhibitor, ensures high sensitivity and specificity in detecting cytotoxic effects.
-
Our experienced team provides detailed data analysis, offering valuable insights into the safety and efficacy of tested compounds.
Our specialized expertise ensures rigorous evaluation of compound safety profiles, crucial for advancing neurotherapeutic research and drug development. Contact us today to discover how our SH-SY5Y-based cytotoxicity safety screening services can support your scientific objectives.
Reference
-
Dalberto, Daiana, et al. "Cytotoxic and genotoxic evaluation of cotinine using human neuroblastoma cells (SH-SY5Y)." Genetics and Molecular Biology 43 (2020): e20190123.
For Research Use Only | Not For Clinical Use



 Fig.1 Cell viability evaluation of SH-SY5Y after cotinine and nicotine treatment.1
Fig.1 Cell viability evaluation of SH-SY5Y after cotinine and nicotine treatment.1
 Download our brochure
Download our brochure