Proteolysis-targeting chimeric molecules (Protein Degraders) represent an emerging technique that is receiving much attention for therapeutic intervention. The mechanism is based on the inhibition of protein function by hijacking a ubiquitin E3 ligase for protein degradation. Based on our advanced technology platforms and extensive experience, Creative Biolabs has been a long-term expert in the field of Protein Degraders development, and we can provide a variety of ligand design strategies for Protein Degraders projects.
α-Synuclein is a ubiquitous 140-amino acid protein of 18-20 kDa that is encoded by a single gene consisting of seven exons borne by chromosome 4. It is the precursor protein of a non-amyloid b component of senile plaques (NACP) in Alzheimer’s disease (AD). This protein is abundant in neurons, especially enriched in presynaptic terminals and is also found to be the major protein component in the hallmark lesions of Parkinson’s disease (PD), Lewy bodies (LBs) and dystrophic neuritis.
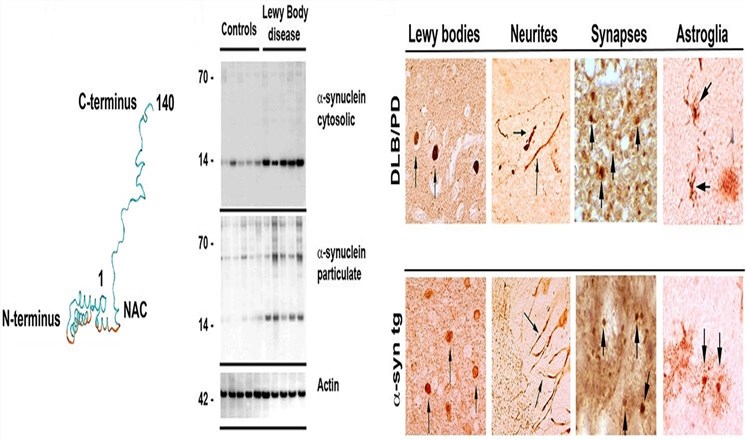 Fig.1 Structure of α-synuclein and its role in neurodegenerative diseases.1
Fig.1 Structure of α-synuclein and its role in neurodegenerative diseases.1
α-Synuclein has an amphipathic lysine-rich amino terminus and a disordered, acidic carboxy-terminal tail. The central region of α-synuclein contains a highly hydrophobic motif that comprises amino acid residues 65-90, known as the non-amyloid-β component of AD amyloid plaques (NAC). The NAC region is indispensable for α-synuclein aggregation; the deletion of large segments within this motif greatly diminishes α-synuclein oligomerization and fibrillogenesis in a cell-based assay. Inhibition of the progress of α-synuclein protein aggregation may be a potential strategy for treating PD and its associated diseases.
α-Synuclein-targeting Protein Degraders is a bifunctional molecule that recruits α-synuclein proteins into proximity with an E3 ubiquitin ligase to trigger target ubiquitination and subsequent proteasomal degradation. Regulating protein function through α-synuclein-targeting Protein Degraders has emerged as a new modality of discovery chemistry with attractive potential both as a tool for α-synuclein target validation and the development of novel therapeutics for neurodegenerative diseases.
Several phenothiazine analogs including the tricyclic ligands SIL23, SIL5, and SIL26 have shown reasonable in vitro selectivity for α-synuclein oligomerization and fibrillogenesis. However, their low in vitro stability and moderate affinity for α-synuclein aggregates limits their utility as Protein Degraders ligands. Therefore, there is a need to identify new chemical scaffolds that can serve as lead compounds for Protein Degraders development. Creative Biolabs can provide modification and engineering of existing ligands, which promotes these poorly selective or weak-affinity ligands to generate functional Protein Degraders as potential selective chemical probes or therapeutics tools.
In addition, based on the specific structural characteristics of the α-synuclein, Creative Biolabs can also use a variety of strategies to generate high-quality ligands, including but not limited to small-molecules with structure-based computational methods for in silico screening, peptides and recombinant antibodies based on phage display. With the structural information, finding optimal ligands with high-throughput techniques facilitates successful Protein Degraders design. Protein Degraders technology provides a promising opportunity to target undruggable disease-causing proteins.
Creative Biolabs has long-term devoted to the development of Protein Degraders; our scientists are confident in offering the best and most suitable ligand design for our customers all over the world. If you are interested in our services, please directly contact us and consult our technical supports for more details.
Reference
Our customer service representatives are available 24 hours a day, from Monday to Sunday. Contact Us

