Compared to RNA viruses, the uniqueness of DNA viruses lies in their possession of either double-stranded or single-stranded deoxyribonucleic acid (DNA). These types of viruses typically integrate their DNA into the host cell's DNA, and then replicate themselves using the host's replication mechanisms within the host cell nucleus. In the field of modern medical science, effectively inhibiting the activity of DNA viruses has become a key research and treatment goal. To address this challenge, Creative Biolabs is proud to offer comprehensive anti-DNA virus efficacy evaluation services. This service is designed for pharmaceutical companies, research institutions, and scientific researchers, aiming to support them in making significant progress and breakthroughs in the field of DNA virus research and drug development.
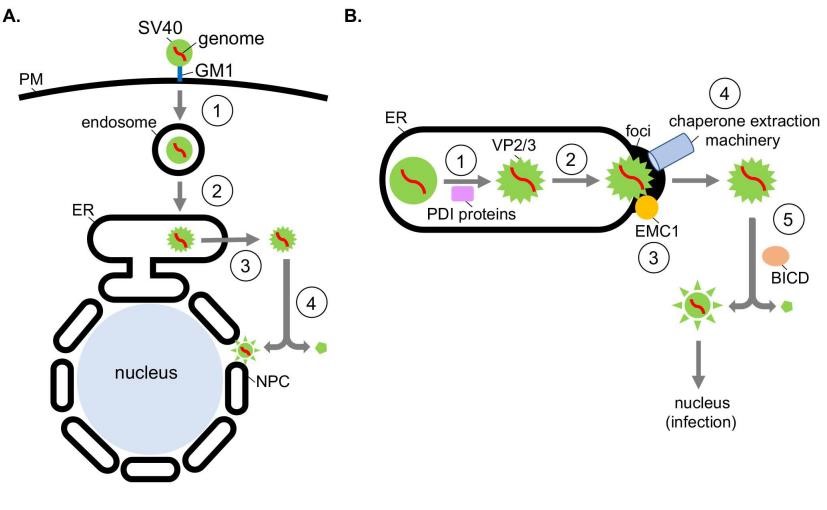 Fig.1 Exploiting ER and cytosolic chaperones during ER escape and disassembly of polyomavirus SV40.1,3
Fig.1 Exploiting ER and cytosolic chaperones during ER escape and disassembly of polyomavirus SV40.1,3
1. Viral Replication Inhibition Experiments
Our laboratory is equipped with advanced equipment and an experienced team of experts, capable of accurately assessing the inhibitory effects of drugs or treatments on DNA virus replication, ensuring that your research and development work can proceed with reliable data support.
2. Time-Dose Response Experiments
For the inhibition of DNA viruses, it is not just about "whether it is effective," but more importantly "how it is effective." Our time-dose response experiments can provide in-depth analysis, helping you understand the specific effects of different time points and drug concentrations on DNA virus replication. Standards of measurement include changes in viral replication levels, host cell survival rate, and the half-maximal inhibitory concentration (IC50). Such detailed analysis can help you optimize drug formulations and treatment methods.
3. Mechanism Validation
In the development of drugs for DNA virus infections, understanding the specific mechanisms of interventions is crucial. Our experts focus not only on the results but also on the process. Through a series of scientifically rigorous experiments, we help you validate the specific mechanisms by which candidate drugs inhibit DNA viruses, thereby deepening the understanding of viral biology.
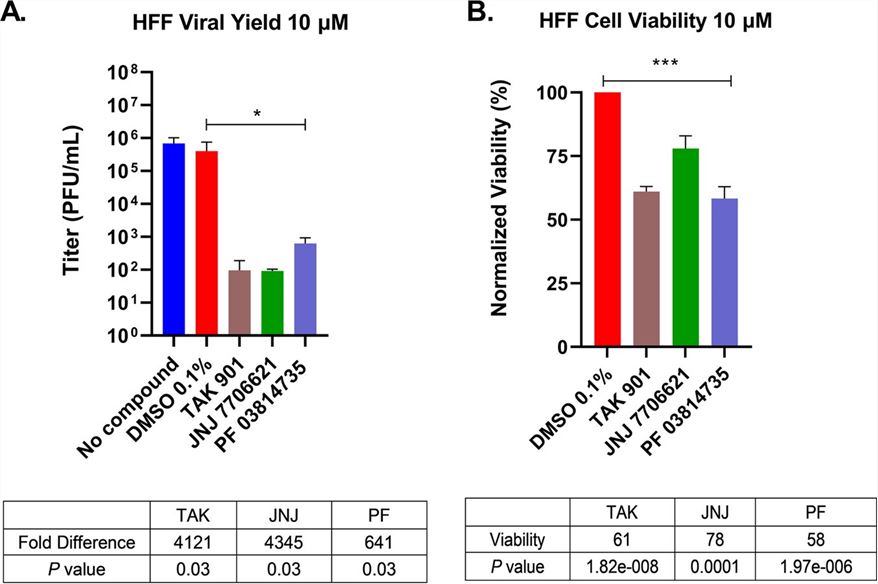 Fig.2 Aurora kinase inhibitors diminished HSV-1 titers in primary cultures of HFFs.2,3
Fig.2 Aurora kinase inhibitors diminished HSV-1 titers in primary cultures of HFFs.2,3
Since its establishment, Creative Biolabs has been committed to the field of biomedical research and drug development. We have cooperated with many well-known pharmaceutical companies and research institutions worldwide, and our project experience has successfully driven a series of infectious disease drug development services. We look forward to working with you to jointly promote the progress of DNA virus research and drug development. Welcome to contact us to discuss the possibility of cooperation.
References: