Anti-RNA Virus Efficacy Evaluation Service for Virus Infection Research
RNA viruses, characterized by their unique RNA genetic material, replicate their genetic information using specialized RNA polymerases. Unlike DNA viruses that replicate within the cell nucleus, RNA viruses primarily replicate in the cytoplasm of the host cell. These viruses mutate rapidly, mainly due to their polymerase lacking a proofreading mechanism, allowing them to quickly adapt to new environments and evade the immune system. Notably, some RNA viruses, like HIV, can reverse transcribe into DNA and integrate into the host genome. In light of this, Creative Biolabs has launched a series of candidate drug evaluation services targeting RNA virus infections, aimed at assessing the potential effects of these drugs against RNA viruses.
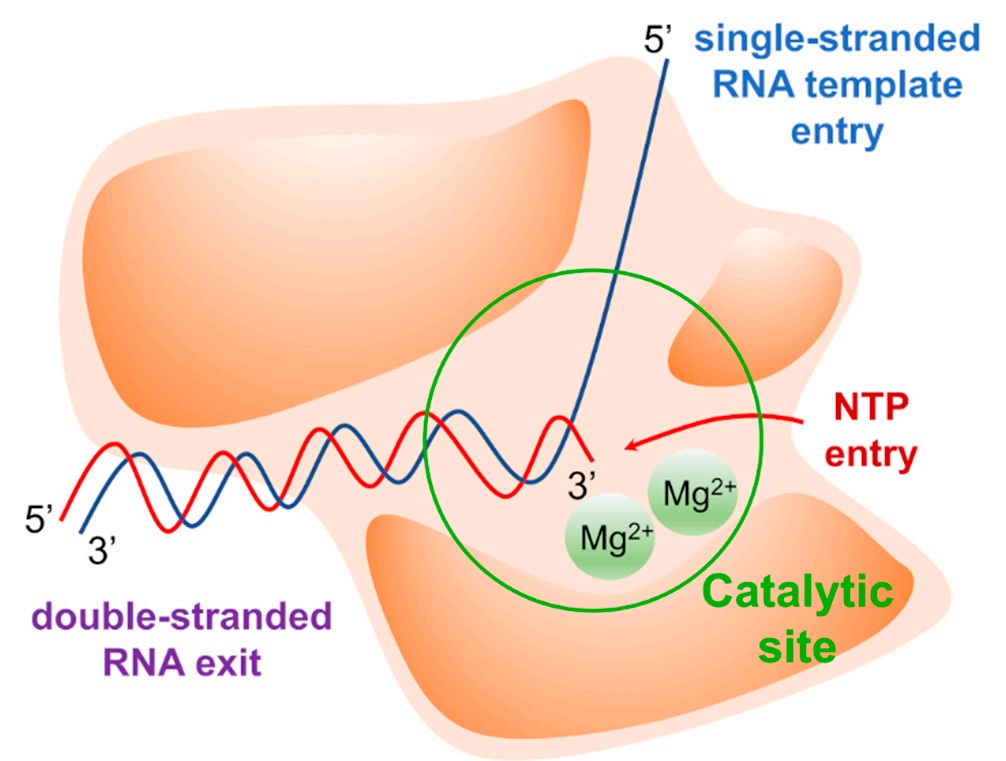 Fig.1 Graphical representation of the RNA-dependent RNA polymerase (RdRp).1,2
Fig.1 Graphical representation of the RNA-dependent RNA polymerase (RdRp).1,2
Key Technical Service for Evaluating the Effectiveness Of Anti-RNA Virus Drug Candidates
Our team demonstrates exceptional expertise in the activity assay of RNA-dependent RNA polymerase (RdRp), a core enzyme in the replication of RNA viruses and a critical target in antiviral drug development. Our services aim to precisely assess the impact of drugs on this key enzyme, providing vital experimental data support for drug development.
1. RdRp Preparation:
-
Virus Culture: We use advanced cell culture techniques to carefully cultivate cells infected with the target RNA virus, ensuring high activity and stability of the starting material.
-
RdRp Extraction: RdRp is precisely extracted from viral particles or infected cells, using efficient separation techniques to ensure the enzyme's purity and activity.
2. Activity Assay:
-
Substrate Preparation: Special labeled RNA templates, such as radioactive or fluorescent labels, are used to ensure the reliability and accuracy of the experimental results.
-
Reaction System Setup: The reaction of RdRp with RNA templates is conducted under optimally controlled conditions, such as specific temperature, pH, and ion concentrations.
-
Drug Treatment: Different concentrations of antiviral drugs are meticulously added to the reaction system containing RdRp and RNA templates to precisely control experimental conditions.
-
Reaction Termination: The reaction is accurately terminated at the appropriate time using efficient termination buffers or heat treatment methods.
4. Result Detection:
-
Electrophoresis Analysis: Advanced gel electrophoresis and autoradiography techniques are used to precisely detect RNA synthesis products.
-
Fluorescence Detection: High-end devices such as fluorescence spectrophotometers or fluorescence microscopes are used to analyze the fluorescently labeled reaction products in detail.
-
Quantitative Analysis: The RNA synthesis amounts in the treatment and control groups are compared to quantitatively assess the specific impact of the drug on RdRp activity.
5. Data Analysis:
-
Inhibition Rate Calculation: Based on the amount of synthesized RNA, the inhibition rate of the drug on RdRp activity is precisely calculated.
-
Dose-Response Curve Plotting: Clear curves are plotted based on the inhibition rates at different drug concentrations, providing accurate evidence for determining the drug's half-maximal inhibitory concentration (IC50).
6. Repetition and Verification:
-
Repeat Experiments: Multiple rounds of strict experiments are conducted to ensure the reliability and reproducibility of the data.
-
Validation Experiments: Experiments are carried out in different host cells to comprehensively assess the universality of the results.
Other Technical Services
-
Functional Analysis of RNA Virus Structural and Non-structural Proteins: Proteomics technologies such as Western blotting and mass spectrometry are used to detect the effects of drugs on the virus's structural and non-structural proteins.
-
Cell Viability Analysis: Cell viability assays, such as MTT assays, are used to evaluate the therapeutic effects of drugs on host cells.
-
Quantitative Analysis of Antiviral Effects: Methods such as virus titer determination and fluorescent quantitative PCR are used to quantify the inhibitory effects of drugs on viral replication.
Creative Biolabs, with its rigorous experimental procedures and precise data analysis, provides indispensable scientific support for clients in the development of anti-RNA virus drugs. Our team not only focuses on the precise execution of experiments but also commits to providing comprehensive and reliable scientific data to support the success of drug development. If you are looking for a reliable partner to evaluate the potential effects of your anti-RNA virus candidate drugs, please contact us.
References:
-
Picarazzi, F.; et al. Targeting the RdRp of emerging RNA viruses: the structure-based drug design challenge. Molecules. 2020, 25(23).
-
under Open Access license CC BY 4.0, without modification.
For Research Use Only. We do not provide direct services or products for patients.
Related Services:
 Fig.1 Graphical representation of the RNA-dependent RNA polymerase (RdRp).1,2
Fig.1 Graphical representation of the RNA-dependent RNA polymerase (RdRp).1,2