Antivirus Efficacy Evaluation Services for Virus Infection Research
In the modern medical field, viral infections have always been a formidable challenge. From influenza and hepatitis C to Ebola hemorrhagic fever, AIDS, and SARS, these malignant infectious diseases not only severely threaten human health, but also make existing drugs and vaccines less effective due to their high mutation rates. Therefore, the development of new antiviral drugs is particularly urgent. Against this backdrop, Creative Biolabs, with its profound accumulation in the field of virology, provides comprehensive antiviral drug efficacy evaluation services, aiming to advance the development of antiviral drugs.
Necessity of Antiviral Efficacy Evaluation
-
Given the high variability of viruses, a comprehensive evaluation of the antiviral effects of candidate drugs is an essential step to ensure their long-term effectiveness and safety.
-
Evaluating antiviral drugs with different mechanisms helps to better understand the principles of drug action, providing critical data support for subsequent clinical applications and drug improvement.
-
Rigorous antiviral efficacy evaluation accelerates the drug development process, offering more possibilities for the prevention and treatment of viral infections.
Antiviral Efficacy Evaluation Services at Creative Biolabs
Creative Biolabs is dedicated to providing comprehensive and diverse efficacy evaluation services for antiviral drug development, aimed at offering clients in-depth scientific data support and a reliable research foundation.
-
Specific Antiviral Drug Efficacy Evaluation:
-
Comprehensive Antiviral Efficacy Evaluation Models:
-
Cell Models: We use various host cells (such as human and animal cells) and specially modified cells for virus infection and drug treatment experiments to simulate the environment within an actual organism.
-
Animal Models: Mice, rats, rabbits, and specific viral infection models (e.g., mouse models of influenza virus infection) are widely used to study the antiviral effects of drugs in the whole organism.
-
Genetic Engineering Models: Transgenic or gene knockout models are used to delve into the role of specific genes in viral infection and drug action.
-
Multifaceted Antiviral Efficacy Evaluation Methods:
-
Viral Titer Determination: Methods like Plaque Forming Unit (PFU) and Tissue Culture Infectious Dose 50% (TCID50) are used to quantify the level of virus replication in cells.
-
Drug Sensitivity Testing: Determines the inhibitory effect of different drug concentrations on viral replication and calculates the drug's half-maximal effective concentration (EC50).
-
Cytotoxicity Testing: Uses MTT/MTS, Lactate Dehydrogenase (LDH) release, etc., to assess the drug's toxicity to host cells.
-
Molecular Biology Techniques: Uses real-time PCR, Western blotting, ELISA, etc., for quantitative analysis of viral RNA/DNA, viral proteins, and host cell responses.
-
Drug Mechanism of Action Research: Combines proteomics, transcriptomics analysis, etc., to reveal the drug's mechanism of action and the affected biological pathways.
-
Pathological Analysis: Uses tissue sectioning and immunohistochemistry in animal models to thoroughly evaluate drug efficacy and tissue toxicity.
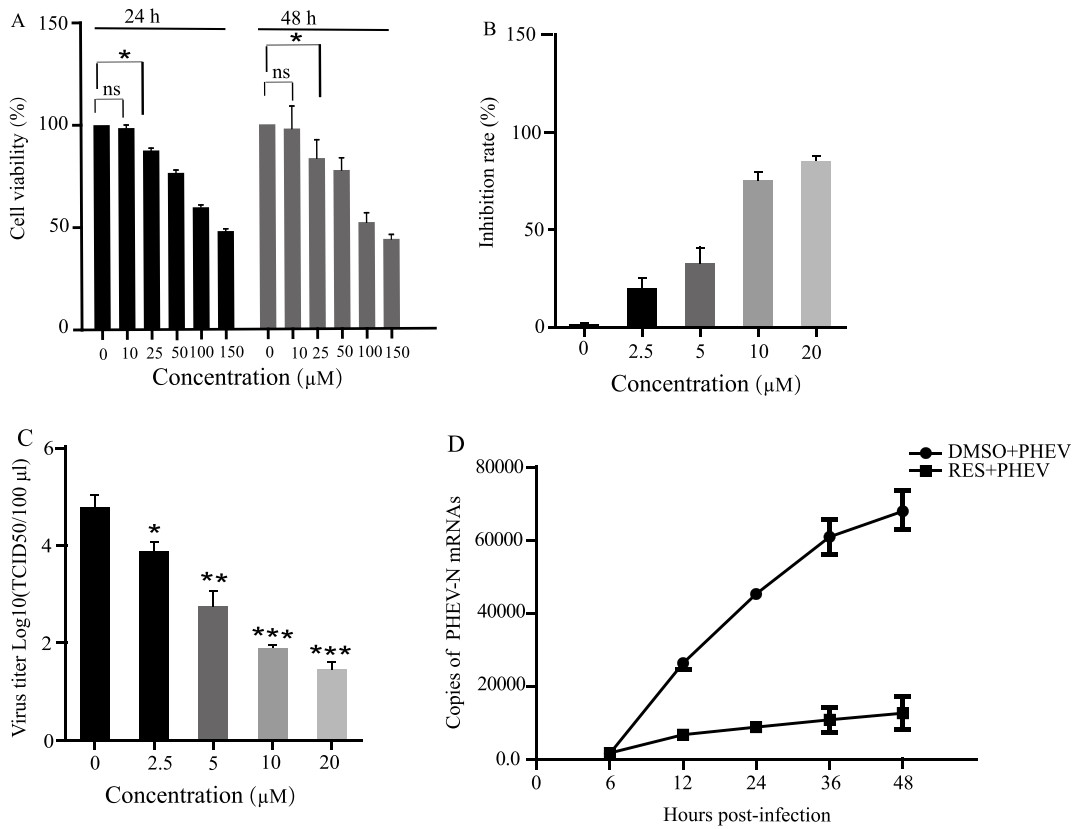 Fig.1 Cytotoxicity and antiviral activity of resveratrol against Porcine hemagglutinating encephalomyelitis virus in vitro.1,2
Fig.1 Cytotoxicity and antiviral activity of resveratrol against Porcine hemagglutinating encephalomyelitis virus in vitro.1,2
Creative Biolabs aims to help researchers better understand and develop new antiviral therapies through precise experimental models and advanced evaluation methods. Our expertise and breadth in technology, along with efficient, considerate, and cost-effective service processes and customer experience, are key to winning customer trust. If you are interested in our services or have more needs, please feel free to contact us.
References:
-
Liu, Y.; et al. Suppression of porcine hemagglutinating encephalomyelitis virus replication by resveratrol. Virology Journal. 2022, 19(1):226.
-
under Open Access license CC BY 4.0, without modification.
For Research Use Only. We do not
provide direct services or products for patients.
Related
Services:
 Fig.1 Cytotoxicity and antiviral activity of resveratrol against Porcine hemagglutinating encephalomyelitis virus in vitro.1,2
Fig.1 Cytotoxicity and antiviral activity of resveratrol against Porcine hemagglutinating encephalomyelitis virus in vitro.1,2