Hybridoma based Antibody Development Service
Creative Biolabs is dedicated to providing efficient and customized monoclonal antibody development services for infectious disease research. Utilizing advanced hybridoma technology, our expert team can efficiently produce monoclonal antibodies with high specificity and affinity against various infectious disease pathogens, aiding clients in making breakthroughs in the fields of infectious disease research and treatment.
Introduction to Hybridoma Technology
Hybridoma technology, a classic and efficient method for preparing monoclonal antibodies, aims to create hybridoma cells with unlimited proliferation capabilities that can stably and continuously produce specific antibodies. The key to this technology is to first stimulate experimental animals to produce specific antibodies using carefully selected immunogens. Then, immune cells extracted from these animals are fused with a specific myeloma cell line. The resulting hybridoma cells not only have continuous proliferation characteristics but also stably produce the target antibodies. In a professional laboratory culture environment, these hybridoma cells are expanded to effectively produce large quantities of the required monoclonal antibodies.
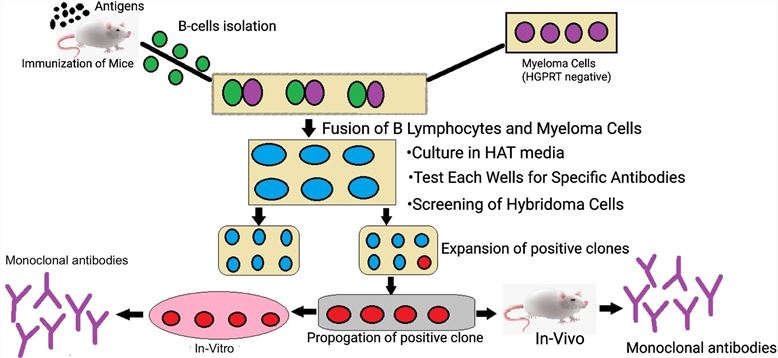 Fig.1 Diagram showing the production of monoclonal antibodies.1,2
Fig.1 Diagram showing the production of monoclonal antibodies.1,2
Hybridoma-based Antibody Development Process
-
Immunogen Selection and Design: For specific infectious disease pathogens, such as viruses, bacteria, or parasites, immunogens are meticulously designed and selected. Based on the specific needs of our clients, we choose the most suitable type of immunogen, such as proteins, peptides, carbohydrates, etc., ensuring their purity and quality.
-
Animal Immunization: Using a carefully designed immunization plan, the immunogen is injected into specially bred experimental animals to stimulate the production of specific antibodies. We monitor the animals' immune responses and perform multiple booster immunizations at appropriate times to achieve optimal immunization effects.
-
Cell Fusion and Hybridoma Screening: Immune cells, such as splenocytes, are extracted from immunized animals and fused with myeloma cell lines to form hybridoma cells. We employ advanced and efficient electrofusion technology and multiple rounds of screening processes to enhance fusion rates and the efficiency of positive hybridoma cell line selection.
-
Culture and Expansion of Hybridoma Clones: The selected hybridoma cell lines are cultured and expanded to produce the required monoclonal antibodies on a large scale. Our culture conditions are meticulously designed to ensure high-density growth and high antibody yield.
-
Antibody Purification and Quality Control: Monoclonal antibodies are extracted from the culture supernatant and undergo stringent purification and quality control steps to ensure the purity and activity of the antibodies. We use various purification techniques, such as protein A/G affinity chromatography, ion exchange chromatography, etc., to obtain high-purity monoclonal antibodies.
-
Antibody Verification and Functional Analysis: The produced monoclonal antibodies are comprehensively verified and functionally tested using various biochemical and molecular biology techniques, such as ELISA, neutralization assays, etc.
Service Features
-
Advanced Electofusion Technology: Electofusion aligns cells in a string-like formation using high-frequency alternating current voltage to achieve precise point contact, followed by square wave pulses to precisely perforate the contact points of the cell membranes. This technology's advantage lies in its high fusion rate and low damage to cells, ensuring healthy growth and proliferation of hybridoma cells after fusion.
-
High Success Rate: Combining our strict screening program, including at least 2 to 3 rounds of limited dilution, we effectively increase the probability of obtaining stable and high-quality hybridoma cell lines. This process not only improves the accuracy of judgment but also helps eliminate genetically unstable cell lines, ensuring that the final monoclonal antibodies have high specificity and stability.
-
Optional Hybridoma Sequencing Service: We provide comprehensive hybridoma cell sequencing services, including antibody variable region sequencing or full-length gene sequencing, offering clients the opportunity to deeply understand and verify the molecular structure and functionality of their monoclonal antibodies, further ensuring the high quality and applicability of the antibody products.
Creative Biolabs focuses on providing specialized monoclonal antibody development services for infectious disease research through precise hybridoma construction and screening. Our services, backed by an advanced technology platform, a professional team of industry experts, and a rigorous quality control system, offer customized, high-quality monoclonal antibody solutions to research institutions and biopharmaceutical companies worldwide. If you are looking for an efficient and reliable monoclonal antibody development partner, please feel free to contact us. Our expert team will work closely with you, engaging in in-depth discussions to ensure the success of each project.
Reference:
-
Mitra, S.; Tomar, PC. Hybridoma technology; advancements, clinical significance, and future aspects. Journal of Genetic Engineering and Biotechnology. 2021. 19(1):159.
-
under Open Access license CC BY 4.0, without modification.
For Research Use Only. We do not
provide direct services or products for patients.
Related
Services:
 Fig.1 Diagram showing the production of monoclonal antibodies.1,2
Fig.1 Diagram showing the production of monoclonal antibodies.1,2