Introduction to CD3
CD3 is an abbreviation for the cluster of differentiation 3, also known as T3 or the T-cell receptor complex. CD3 comprises a group of proteins expressed on the surface of T cells, a type of white blood cell crucial to the immune system. Consisting of four different polypeptide chains—CD3γ, CD3δ, CD3ε, and CD3ζ—encoded by distinct genes on various chromosomes, CD3 forms a complex with the T-cell receptor (TCR). This recognition complex identifies antigens presented by other cells, transmitting signals from the TCR to the interior of the T cell. This activation leads to various cellular functions such as proliferation, differentiation, cytokine production, and cytotoxicity. CD3 is a potential target for immunotherapy due to its modulation of T cell activity and specificity. For instance, bispecific antibodies binding to both CD3 and a tumor-associated antigen can redirect T cells to eliminate cancer cells.
Introduction to HER2
HER2, short for human epidermal growth factor receptor 2 (also known as ERBB2 or neu), is a protein present on the surface of all breast cells, playing a role in normal cell growth. Encoded by a gene on chromosome 17, HER2 belongs to a receptor family that can activate diverse signaling pathways within the cell. HER2 can form complexes with other family members like HER3 and HER4, triggering cell proliferation, differentiation, survival, and migration. HER2 is a potential target for breast cancer therapy, as it may be overexpressed or amplified in 15% to 20% of breast tumors, termed HER2-positive breast cancers. These cancers tend to be more aggressive and resistant to hormone therapy but more responsive to drugs targeting HER2, such as trastuzumab, pertuzumab, lapatinib, and ado-trastuzumab emtansine.
Signaling Pathways Involved in Bispecific Antibodies Targeting CD3 and HER2
Bispecific antibodies targeting CD3 and HER2 are a type of immunotherapy that can recruit and activate T cells to eliminate HER2-positive tumor cells. CD3 is a protein complex expressed on the surface of T cells, involved in T cell receptor (TCR) signaling. HER2 is a receptor tyrosine kinase overexpressed or amplified in some types of cancer, such as breast, gastric, and ovarian cancer, associated with poor prognosis and therapy resistance. By binding to both CD3 and HER2, bispecific antibodies can induce the formation of a synapse between T cells and tumor cells, leading to the activation of both TCR and HER2 signaling pathways. The TCR signaling pathway activates when T cells recognize antigens on other cells, stimulating various functions of T cells, such as cytokine production and cytotoxicity. The HER2 signaling pathway activates when HER2 receptors bind to growth factors or other receptors, promoting various processes of tumor cells, such as cell survival and migration. Simultaneously engaging CD3 on T cells and HER2 on tumor cells, bispecific antibodies trigger both TCR and HER2 signaling pathways synergistically. Cross-linking of CD3 by bispecific antibodies lowers the threshold for TCR activation, overcoming the requirement for MHC-restricted antigen presentation. Co-stimulation of HER2 by bispecific antibodies enhances the expression of co-stimulatory molecules on tumor cells, increasing the sensitivity of tumor cells to T cell-mediated killing. The combination of these two signaling pathways results in a potent antitumor response that can eliminate HER2-positive tumor cells.
Clinical Status of Bispecific Antibodies Targeting CD3 and HER2
Bispecific antibodies (BsAbs) targeting CD3 and HER2, a type of immunotherapy, can recruit and activate T cells to eliminate HER2-positive tumor cells. Currently, only one BsAb targeting CD3 and HER2, called ertumaxomab, has received market approval. Several other BsAbs targeting CD3 and HER2 are at different stages of clinical trials. Ertumaxomab, approved by the China Food and Drug Administration (CFDA) in 2015, is used for treating HER2-positive metastatic breast cancer after trastuzumab-based therapy failure. It is a trifunctional BsAb binding to Fcγ receptors on accessory immune cells, enhancing the antitumor response. Ertumaxomab has demonstrated promising efficacy and safety in clinical trials, with an overall response rate of 33% and a median progression-free survival of 4.5 months.
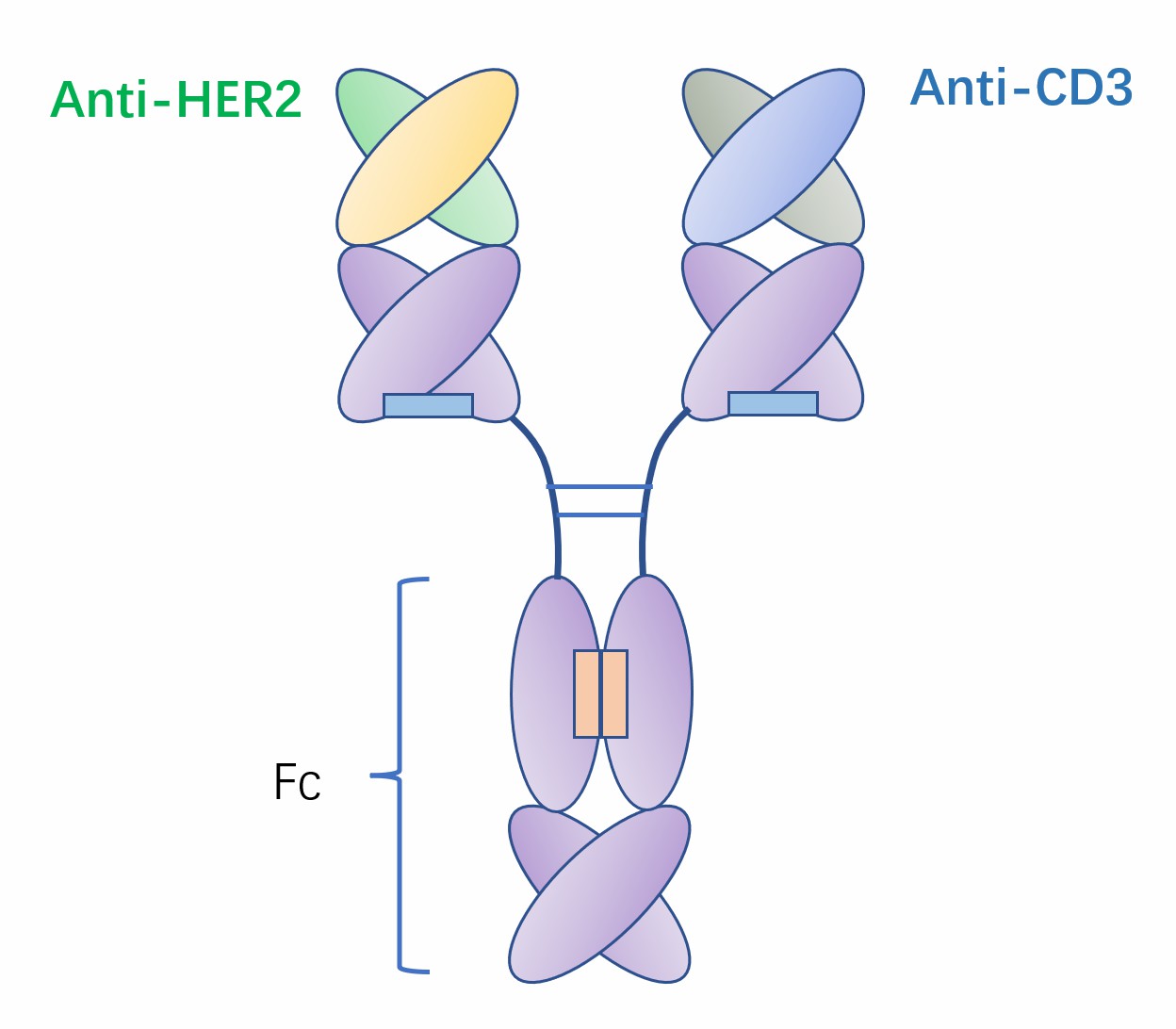
Fig.1 Schematic diagram of ertumaxomab (Creative Biolabs)
Table 1. Example of bispecific antibodies targeting CD3 and HER2
|
Name
|
Target
|
Clinical phase
|
Indication
|
Population
|
|
Ertumaxomab
|
CD3/HER2
|
Approved
|
Breast cancer
|
HER2-positive metastatic breast cancer after failure of trastuzumab-based therapy
|
One of the BsAbs targeting CD3 and HER2 that are in clinical trials is GBR 1302. By simultaneously binding to CD3 of T cells and HER2 of tumor cells, GBR 1302 can induce the formation of synapses between T cells and tumor cells, thereby activating the TCR and HER2 signaling pathways, leading to T cell-mediated killing of tumor cells. GBR 1302, built using Glenmark's BEAT® platform, is currently in a Phase I/II clinical trial (NCT03084237) for the treatment of patients with HER2-positive or allele-amplified solid tumors.
References
1. Griffiths PD. An antibody which behaves like a man with a wife and a mistress. Rev Med Virol. 2009 Jul;19(4):181-3.
2. Yu S, et al. A novel asymmetrical anti-HER2/CD3 bispecific antibody exhibits potent cytotoxicity for HER2-positive tumor cells. J Exp Clin Cancer Res. 2019 Aug 14;38(1):355.
3. Ma J,et al. Bispecific Antibodies: From Research to Clinical Application. Front Immunol. 2021 May 5;12:626616.
4. Kontermann RE, Brinkmann U. Bispecific antibodies. Drug Discov Today. 2015 Jun;20(7):838-47.
5. Spiess C, et al. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol. 2015 Oct;67(2 Pt A):95-106.
6. Wu X, et al. Advances in engineering of high affinity bispecific antibodies. Protein Cell. 2019 Apr;10(4):241-62.
7. Fan G, et al. Bispecific antibodies and their applications. J Hematol Oncol. 2015 Dec 29;8:130.
8. Lewis SM, et al. Generation of bispecific IgG antibodies by structure-based design of an orthogonal Fab interface. Nat Biotechnol. 2014 Feb;32(2):191-8.
9. Schaefer W, et al. Immunoglobulin domain crossover as a generic approach for the production of bispecific IgG antibodies. Proc Natl Acad Sci U S A. 2011 Jul 12;108(28):11187-92.
10. Labrijn AF, et al. Therapeutic IgG4 antibodies engage in Fab-arm exchange with endogenous human IgG4 in vivo. Nat Biotechnol. 2009 Sep;27(9):767-71.
11. Klein C, et al. Progress in overcoming the chain association issue in bispecific heterodimeric IgG antibodies. MAbs. 2009 Nov-Dec;1(6):557-63.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY










