Introduction to DLL4
DLL4 is an abbreviation for Delta-like ligand 4, also known as delta-like canonical Notch ligand 4. It is a transmembrane ligand protein mainly expressed on arterial endothelial cells and capillaries, encoded by the DLL4 gene. DLL4 binds to Notch receptors, activates the Notch signaling pathway, and regulates the differentiation, proliferation, migration, and angiogenesis of endothelial cells. DLL4 plays a crucial role in tumor angiogenesis by inhibiting the branching and differentiation of endothelial cells, leading to abnormal proliferation, distortion, immaturity, and low perfusion of tumor vessels. DLL4 is a potential target for various solid tumors, including breast cancer, colon cancer, ovarian cancer, lung cancer, gastric cancer, etc. The overexpression of DLL4 is associated with the invasiveness, metastasis, and prognosis of tumors.
Introduction to VEGF
VEGF is an abbreviation for vascular endothelial growth factor, also known as vascular permeability factor (VPF). It is a signal protein secreted by various cells that can stimulate angiogenesis. VEGF belongs to the cystine-knot growth factor family, binding to VEGFRs, activating the VEGF/VEGFR signaling pathway, and regulating vasculogenesis (the formation of the embryonic circulatory system) and angiogenesis (the growth of new blood vessels from existing vessels). VEGF is a major driving factor of tumor angiogenesis, restoring tissue oxygen supply under hypoxic conditions. It also increases vascular permeability, facilitating tumor cells to escape from immune system attack. VEGF is a potential target for various solid tumors, including renal cell carcinoma, neuroendocrine tumors, thyroid cancer, liver cancer, etc. The overexpression of VEGF is associated with tumor growth, metastasis, and prognosis.
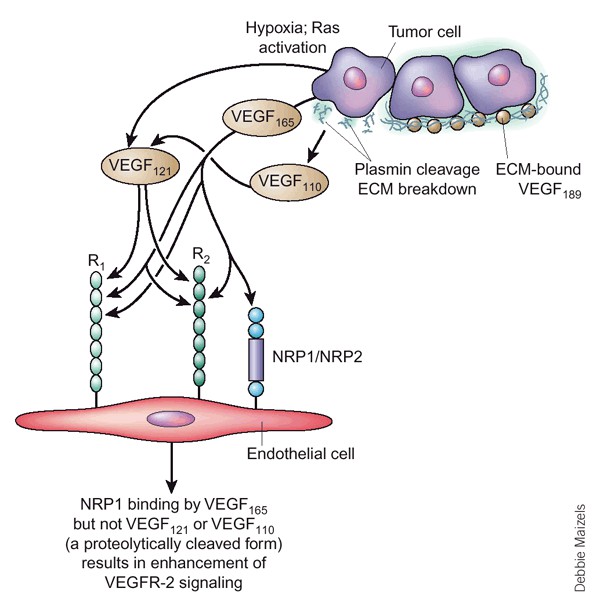
Fig.1 The VEGF isoforms and their interaction with VEGF receptors (Ferrara N, 2003)
Signaling Pathways Involved in Bispecific Antibodies Targeting DLL4 and VEGF
Targeting DLL4 and VEGF involves two major signaling pathways: the Notch signaling pathway and the VEGF/VEGFR signaling pathway. The Notch signaling pathway is an essential mechanism of cell-to-cell communication involved in various biological processes, such as cell fate determination, proliferation, differentiation, migration, and apoptosis. The activation of the Notch signaling pathway depends on the interaction between ligands and receptors, among which DLL4 is one of the ligands for Notch1 and Notch4. The VEGF/VEGFR signaling pathway is a crucial mechanism of angiogenesis regulation, involved in various physiological and pathological processes, such as embryonic development, tissue repair, inflammation, and tumor growth. The activation of the VEGF/VEGFR signaling pathway depends on the binding of VEGF and VEGFRs, among which VEGF-A is one of the most common and studied members of the VEGF family. Targeting DLL4 and VEGF can enhance the anti-tumor effects through synergistic anti-angiogenic mechanisms. DLL4 inhibitors can induce excessive proliferation of dysfunctional vessels that cannot support tumor growth, while VEGF inhibitors can reduce the number of vessels and decrease the blood supply to tumors. Simultaneously targeting DLL4 and VEGF can inhibit the feedback regulation of both signaling pathways, avoiding resistance to single-target therapy.
Clinical Status of Bispecific Antibodies Targeting DLL4 and VEGF
Currently, several bispecific antibodies targeting DLL4 and VEGF have entered clinical trials, mainly developed by ABL Bio Inc., OncoMed Pharmaceuticals Inc., Novartis AG, and other companies or institutions. These bispecific antibodies adopt different molecular formats, such as IgG2, IgG1, scFv-Fc, etc., to achieve high affinity and specificity for both targets. These bispecific antibodies mainly target various solid tumors, including ovarian cancer, colon cancer, gastric cancer, pancreatic cancer, lung cancer, etc. Some trials also combine bispecific antibodies with chemotherapy or targeted therapy to improve therapeutic effects. So far, no bispecific antibody targeting DLL4 and VEGF has been approved for marketing, but some clinical trials have shown their good safety and efficacy, offering more treatment options for cancer patients. Table 1 summarizes the clinical status of some bispecific antibodies targeting DLL4 and VEGF.
Table 1. Bispecific antibodies targeting DLL4 and VEGF in clinical trials.
|
Bispecific antibody
|
Format
|
Targets
|
Indications
|
Phase
|
Sponsor
|
|
ABL001/ NOV1501/ TR009
|
IgG2
|
DLL4/VEGF
|
Solid tumors
|
I
|
ABL Bio Inc./ Novartis AG
|
|
Navicixizumab/ OMP-305B83
|
IgG1
|
DLL4/VEGF
|
Ovarian cancer, colorectal cancer, gastric cancer
|
I/II
|
OncoMed Pharmaceuticals Inc./ Mereo BioPharma Group plc
|
References
1. Yeom DH, et al. ABL001, a Bispecific Antibody Targeting VEGF and DLL4, with Chemotherapy, Synergistically Inhibits Tumor Progression in Xenograft Models. Int J Mol Sci. 2020 Dec 29;22(1):241.
2. Cui X, et al. A Novel Bispecific Antibody Targeting PD-L1 and VEGF With Combined Anti-Tumor Activities. Front Immunol. 2021 Dec 2;12:778978.
3. Zhang X, et al. Targeting the DLL/Notch Signaling Pathway in Cancer: Challenges and Opportunities. Mol Cancer Ther. 2020 Jan;19(1):3-15.
4. Li Y, et al. Abstract C164: Dual targeting of the DLL4 and VEGF pathways with a bispecific monoclonal antibody inhibits tumor growth in preclinical models. Mol Cancer Ther. 2015 Dec;14(12 Supplement 2):C164.
5. Hanahan D, et al. Hallmarks of cancer: the next generation. Cell. 2011 Mar 4;144(5):646-74.
6. Ferrara N, et al. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov. 2016 Jun;15(6):385-403.
7. Ellis LM, et al. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008 Aug;8(8):579-91.
8. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012 Mar 22;12(4):252-64.
9. Benedito R, et al. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009 May 29;137(6):1124-35.
10. Shibuya M, et al. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006 May 15;312(9):549-60.
11. Ferrara N, et al. The biology of VEGF and its receptors. Nat Med. 2003 Jun;9(6):669-76.
12. Noguera-Troise I, et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006 Dec 21;444(7122):1032-7.
13. Ridgway J, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006 Dec 21;444(7122):1083-7.
14. Lee SH, et al. Next-IO™ VEGF × DLL4 Therapeutic Bispecific Antibody Program [abstract]. In: Proceedings of the American Association for Cancer Research Annual Meeting 2019; 2019 Mar 29-Apr 3; Atlanta, GA: AACR; Cancer Res 2019;79(13 Suppl):Abstract nr LB-057.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY










