Introduction of PD1
PD1, also known as programmed cell death protein 1 or CD279 or PDCD1, is an immunosuppressive receptor expressed on the surface of T cells, B cells, NK cells, and other immune cells. It is encoded by the PDCD1 gene on human chromosome 2. The PD1 protein consists of 288 amino acids and includes a signal peptide, an immunoglobulin superfamily V domain, a transmembrane region, and a cytoplasmic region. PD1's main biological function is to inhibit the activation and proliferation of T cells by binding to its ligands PD-L1 or PD-L2. This helps maintain immune tolerance and prevent autoimmune reactions. PD1 is highly expressed in various tumors, leading to T cell exhaustion and tumor immune evasion. As a result, it is considered a potential therapeutic target for various solid tumors and hematological malignancies.
Introduction of PDL1
PDL1, also known as programmed death-ligand 1 or CD274 or B7-H1, is an immunosuppressive ligand that binds to its receptor PD-1 expressed by T cells and other immune cells. This binding helps regulate immune responses and prevents excessive activation and autoimmunity. Many tumors exploit this mechanism by overexpressing PDL1, which is often associated with a poor prognosis. Recently, some tumors have also been found to express PD-1. In tumors, PDL1 binding to PD-1 on immune cells promotes immune evasion and tumor progression by inhibiting the function of cytotoxic T lymphocytes. PDL1/PD-1-targeted therapy has revolutionized cancer treatment and is often the first-line treatment for some cancers, as it can promote durable anti-tumor immune responses in select patients with advanced cancers. However, some patients do not respond well to this therapy or develop resistance. The exact mechanisms for this are still unclear. This review will discuss the current status of PDL1/PD-1-targeted therapy, the oncogenic expression of PDL1, the emerging tumor-intrinsic roles of PDL1 and its receptor PD-1, and how they may contribute to tumor progression and immunotherapy responses in different oncology models.
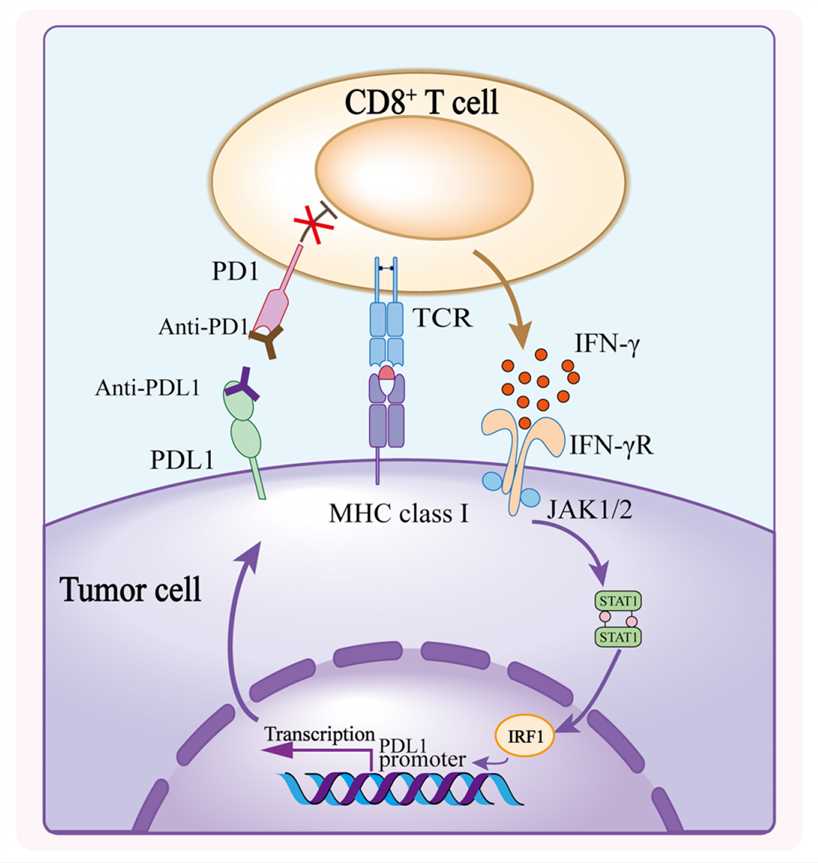
Fig.1 Mechanism of PD1/PDL1 Blockade (Lei Q, 2020)
Signaling Pathways Involved in Bispecific Antibodies Targeting PD1 and PDL1
Bispecific antibodies that target PD1 and PDL1 are a new type of immune checkpoint inhibitors. These antibodies can simultaneously block the interactions between PD1 and PDL1 or PD-L2, which enhances the killing ability of T cells against tumor cells. They work by targeting several important signaling pathways, such as:
-
One of these pathways is the TCR/CD28 signaling pathway in T cells. The bispecific antibodies can restore the normal activity of this pathway by blocking the binding of PD-1 to its ligands. This promotes T cell proliferation, cytokine secretion, and cytotoxic granule release.
-
Additionally, the antibodies can inhibit the expression of PDL1 on tumor cells. This reduces the immune evasion ability of the tumor cells and increases the possibility of tumor cell recognition and elimination by T cells.
-
Furthermore, the bispecific antibodies can modulate the ratio of immune cells in the tumor microenvironment. This improves the immune suppression state and enhances the efficiency and durability of the immune response.
Clinic Status of Bispecific Antibodies Targeting PD1 and PDL1
In terms of their clinical status, bispecific antibodies targeting PD1 and PDL1 have shown good safety and efficacy in clinical trials. However, none of them have been approved for marketing yet. There are currently many bispecific antibodies targeting PD1 and PDL1 in development or planned for development. Most of these antibodies are in phase I or I/II, with a few in phase II or III. They are being developed by companies and research institutions from various countries, including the United States, China, Japan, South Korea, and Germany. These antibodies mainly target tumor types such as non-small cell lung cancer, melanoma, colorectal cancer, breast cancer, gastric cancer, esophageal cancer, ovarian cancer, and head and neck squamous cell carcinoma. For example, LY3434172 is a bispecific antibody that targets both PD-1 and PD-L1. It simultaneously blocks the interactions between these two immune checkpoints, enhancing the killing ability of T cells against tumor cells. LY3434172 is developed by Eli Lilly and Company and is currently in phase I/II clinical trials, primarily for treating solid tumors. One arm of LY3434172 blocks the binding of PD-1 to PD-L1 and PD-L2, while the other arm blocks the binding of PD-L1 to PD-1 and CD80. LY3434172 has shown promising results in enhancing T cell activation and anti-tumor immunity in both in vitro and in vivo experiments. It is a promising immunotherapy strategy that may offer new treatment options for various refractory tumors.
Bispecific antibodies that target PD1 and PDL1 have emerged as a promising immunotherapy strategy, offering potential new treatment options for refractory tumors. However, these antibodies also come with their own set of challenges. Determining the optimal dose, schedule, combination, biomarker, toxicity, and resistance poses significant obstacles. Therefore, it is crucial to conduct further preclinical and clinical studies to fully understand the potential and limitations of bispecific antibodies targeting PD1 and PDL1.
References
1. Yi M, et al. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer. 2022 Jan 21;21(1):28.
2. Lei Q, et al. Resistance Mechanisms of Anti-PD1/PDL1 Therapy in Solid Tumors. Front Cell Dev Biol. 2020 Jul 21;8:672.
3. Upadhaya S, et al. Challenges and opportunities in the PD1/PDL1 inhibitor clinical trial landscape. Nat Rev Drug Discov. 2022 Jul;21(7):482-483.
4. Kotanides H, et al. Bispecific Targeting of PD-1 and PD-L1 Enhances T-cell Activation and Antitumor Immunity. Cancer Immunol Res. 2020 Oct;8(10):1300-1310.
5. Cui X, et al. A Novel Bispecific Antibody Targeting PD-L1 and VEGF With Combined Anti-Tumor Activities. Front Immunol. 2021 Dec 2;12:778978.
6. Hodge J, Campbell J. Challenges and opportunities in the PD1/PDL1 inhibitor landscape. Nat Rev Drug Discov. 2022 Jan 14.
7. Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002 Feb;2(2):116-26.
8. Keir ME, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704.
9. Ishida Y, et al. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992 Nov;11(11):3887-95.
10. Nishimura H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999 Aug;11(2):141-51.
11. Dong H, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002 Aug;8(8):793-800.
12. Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015 Sep;125(9):3384-91.
13. Topalian SL, et al. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015 Apr 13;27(4):450-61.
14. Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015 Apr 3;348(6230):56-61.
15. Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017 Jan 18;541(7637):321-330.
16. Zou W, et al. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016 Mar 9;8(328):328rv4.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY










