Introduction to EGFR
EGFR stands for epidermal growth factor receptor, a type of protein spanning the cell membrane and acting as a switch for various cellular processes. It is encoded by the EGFR gene, consisting of four main parts: an extracellular domain binding to other molecules, a transmembrane domain anchoring the protein to the membrane, a cytoplasmic domain with enzymatic activity, and a tail domain interacting with other proteins inside the cell. EGFR plays a key role in regulating cell growth, survival, migration, and differentiation. However, when the EGFR gene is altered or overexpressed, it can cause the EGFR protein to malfunction, sending abnormal signals to the cell, leading to uncontrolled cell division and tumor formation. EGFR is implicated in various types of cancer, such as non-small cell lung cancer (NSCLC), colorectal cancer, head and neck cancer, etc.
Introduction to c-MET
c-MET is an acronym for cellular-mesenchymal epithelial transition factor, a type of protein spanning the cell membrane and acting as a receptor for another protein called hepatocyte growth factor (HGF) or scatter factor (SF). It is encoded by the MET gene, located on chromosome 7. c-MET consists of three domains: an extracellular domain binding to HGF, a transmembrane domain anchoring the protein to the membrane, and a cytoplasmic domain with enzymatic activity that interacts with other proteins inside the cell. c-MET is involved in various biological processes such as cell growth, survival, migration, invasion, and differentiation. However, when the MET gene is mutated, amplified, or overexpressed, it can cause the c-MET protein to malfunction, sending abnormal signals to the cell, leading to uncontrolled cell division and tumor formation. c-MET is implicated in various types of cancer, including liver, lung, colon, breast, pancreatic, ovarian, prostate, gastric, and brain cancers.
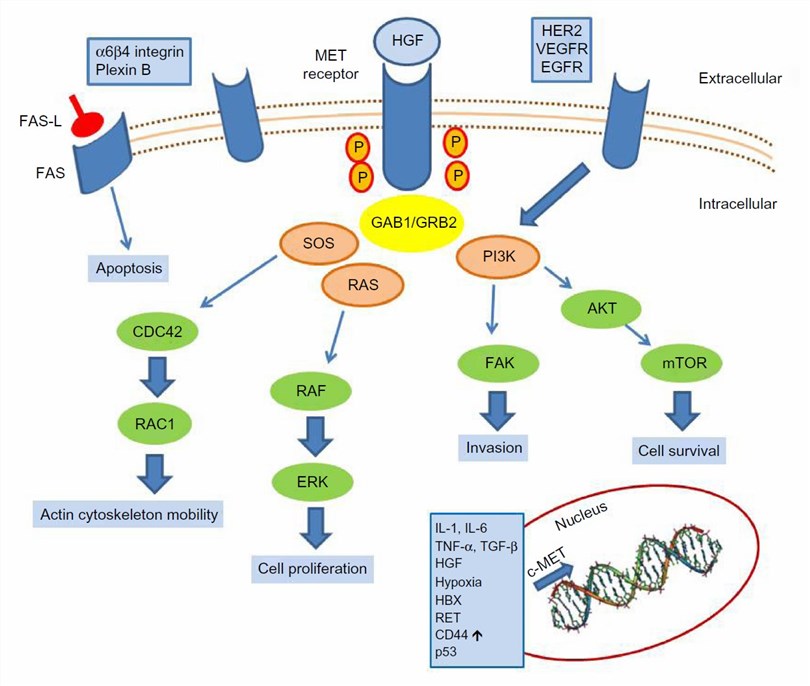
Fig.1 c-MET activation signaling pathways (Granito A, 2015)
Signaling Pathways Involved in Bispecific Antibodies Targeting EGFR and c-MET
The signaling pathways of EGFR and c-MET are crucial for understanding how these receptor tyrosine kinases regulate various cellular processes and contribute to tumor development and progression. EGFR and c-MET are two receptor tyrosine kinases involved in various cellular processes, including cell proliferation, survival, migration, invasion, differentiation, and angiogenesis. They are also implicated in various types of cancer, such as non-small cell lung cancer (NSCLC), colorectal cancer, head and neck cancer, etc. EGFR and c-MET can activate multiple downstream signaling pathways, such as the PI3K/AKT pathway, the Ras/MAPK pathway, the JAK/STAT pathway, and the SRC pathway. These pathways regulate the expression and activity of various genes and proteins that affect the behavior and fate of cancer cells.
Bispecific antibodies targeting EGFR and c-MET are designed to simultaneously block the activity of both receptors and inhibit their signaling pathways. By doing so, they can effectively suppress the growth and survival of cancer cells, induce apoptosis, and overcome resistance to single-targeted therapies. Moreover, bispecific antibodies can recruit immune cells to the tumor site and trigger antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP), further enhancing the anti-tumor effects.
Clinical Status of Bispecific Antibodies Targeting EGFR and c-MET
Bispecific antibodies targeting EGFR and c-MET are a novel class of therapeutics that aim to simultaneously inhibit two key drivers of tumor growth and resistance in various types of cancer, especially non-small cell lung cancer (NSCLC). Several bispecific antibodies targeting EGFR and c-MET have entered clinical trials, mainly developed by Janssen, AstraZeneca, Merus, and other companies or research institutions. The table below summarizes some of the clinical trials of bispecific antibodies targeting EGFR and c-MET.
Table 1. Bispecific antibodies targeting EGFR and c-MET in clinical trails
|
Bispecific antibody
|
Targets
|
Clinical trial phase
|
Indication
|
Trial ID
|
|
JNJ-61186372 (JNJ-372)
|
EGFR/c-MET
|
I/II
|
Advanced NSCLC previously treated with EGFR inhibitors
|
NCT02609776
|
|
AZD9592
|
EGFR/c-MET
|
I/II
|
Advanced NSCLC previously treated with EGFR inhibitors
|
NCT04700966
|
|
MCLA-129
|
EGFR/c-MET
|
I/II
|
Advanced solid tumors, including NSCLC, gastric cancer, colorectal cancer, etc.
|
NCT04014960
|
So far, no bispecific antibody targeting EGFR and c-MET has been approved for marketing, but some single-targeted or multi-targeted antibody drugs have been approved, such as necitumumab (Portrazza) targeting EGFR and tepotinib (Tepmetko) targeting c-MET. These drugs provide reference and inspiration for the development and approval of bispecific antibodies targeting EGFR and c-MET.
References
1. Granito A, et al. c-MET receptor tyrosine kinase as a molecular target in advanced hepatocellular carcinoma. J Hepatocell Carcinoma. 2015 Apr 24;2:29-38.
2. Moores SL, et al. A Novel Bispecific Antibody Targeting EGFR and cMet Is Effective against EGFR Inhibitor–Resistant Lung Tumors. Cancer Res. 2016 Jul 1;76(13):3942-3953.
3. Comer F, et al. Abstract 5736: AZD9592: An EGFR-cMET bispecific antibody-drug conjugate (ADC) targeting key oncogenic drivers in non-small-cell lung cancer (NSCLC) and beyond. Cancer Res. 2021 Apr 1;83(7_Supplement):5736.
4. Chen G, et al. Abstract LB-002: A novel c-Met/EGFR bispecific targeting antibody drug conjugate (ADC) with superior anti-tumor efficacy in vivo. Cancer Res. 2015 Aug 1;75(15_Supplement):LB-002.
5. Ma J, et al. Bispecific Antibodies: From Research to Clinical Application. Front Immunol. 2021 May 5;12:626616.
6. Chiu ML, et al. Discovery and development of an asymmetric IgG-like bispecific antibody targeting EGFR and c-MET, engineered through H-H and H-L chain charge-based heterodimerization. Cancer Res. 2021 Apr 1;83(7_Supplement):5675.
7. Chen Y, et al. Abstract B241: Bispecific antibody targeting EGFR and cMet demonstrates superior activity in cellular downstream signaling compared to the combination of single pathway inhibitors. Mol Cancer Ther. 2013 Nov 1;12(11_Supplement):B241.
8. Zhang Y, et al. The bispecific antibody MCLA-129 impairs NSCLC tumor growth by targeting EGFR and c-MET, inhibiting ligand-induced signaling and promoting ADCC and ADCP [abstract]. In: Proceedings of the American Association for Cancer Research Annual Meeting 2021; 2021 Apr 10-15 and May 17-21. Philadelphia (PA): AACR; Cancer Res 2021;81(13_Suppl):Abstract nr LB071.
9. Sequist LV, et al. Phase Ib Study of JNJ-61186372, a Human Bispecific EGFR and cMet Antibody, in Patients with Advanced Non-Small Cell Lung Cancer (NSCLC). J Thorac Oncol. 2019 Jul;14(7):1249-1257.
10. Herbst RS, et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2015 Jul;16(7):763-774.
11. Paik PK, et al. Tepotinib in Non–Small Cell Lung Cancer with MET Exon 14 Skipping Mutations. N Engl J Med. 2020 Sep 3;383(10):931-943.
Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001 Feb;2(2):127-137.
12. Gherardi E, et al. Targeting MET in cancer: rationale and progress. Nat Rev Cancer. 2012 Feb;12(2):89-103.
13. Zhang Z, et al. Targeting EGFR/c-MET axis by a novel bispecific antibody potently inhibits non-small cell lung cancer resistant to EGFR inhibitors [abstract]. In: Proceedings of the American Association for Cancer Research Annual Meeting 2018; April 14–18, Chicago (IL). Philadelphia (PA): AACR; Cancer Res 2018;78(13_Suppl):Abstract nr 5610.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY










