Introduction to IGF-1
IGF-1, short for Insulin-like Growth Factor 1, also known as Somatomedin C, is a peptide hormone similar to insulin, secreted by the liver and bound to high-affinity binding proteins in the blood. The gene encoding IGF-1 is IGF1, located on human chromosome 12. The protein structure of IGF-1 consists of 70 amino acids, divided into four regions: A, B, C, and D. The A and B regions correspond to the A chain and B chain of insulin, the C region is an extension peptide, and the D region is the C terminus. IGF-1 binds to the IGF-1 receptor (IGF-1R), activating various signaling pathways, such as PI3K/AKT and MAPK/ERK, regulating cell proliferation, differentiation, migration, apoptosis, and metabolism. IGF-1 plays an important role in normal growth and development, but is also associated with various diseases, especially cancer. IGF-1 is considered to be an important promoter of cancer, promoting tumor cell survival, invasion and metastasis, as well as resistance to chemotherapy and radiotherapy. Therefore, IGF-1 is a potential target for many cancer therapies.
Introduction to IGF-2
IGF-2, short for Insulin-like Growth Factor 2, also known as Somatomedin A, is a peptide hormone similar to insulin, mainly produced by the placenta during fetal development and by the liver and other tissues in adulthood. The gene encoding IGF-2 is IGF2, located on human chromosome 11. The protein structure of IGF-2 consists of 67 amino acids, divided into four regions: A, B, C and D. The A and B regions correspond to the A chain and B chain of insulin, the C region is an extension peptide, and the D region is the C terminus. IGF-2 binds to IGF-1 receptor (IGF-1R), insulin receptor (IR) or IR/IGF-1R hybrid receptor (IR-A), activating various signaling pathways, such as PI3K/AKT and MAPK/ERK, regulating cell proliferation, differentiation, migration, apoptosis and metabolism. IGF-2 plays a key role in fetal growth and development, but is also associated with various diseases, especially cancer. IGF-2 is considered to be an important driver of cancer, promoting tumor cell growth, angiogenesis and metastasis, as well as escaping immune surveillance and clearance. Therefore, IGF-2 is also a potential target for many cancer therapies.
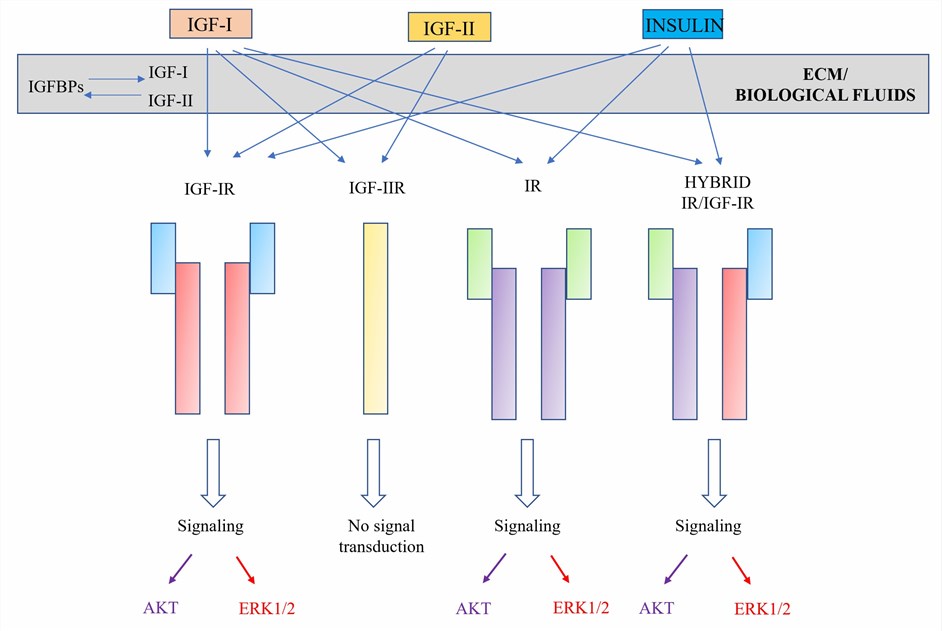
Fig.1 The IGF bio-regulation system (Creative Biolabs)
Signaling Pathways Involved in Bispecific Antibodies Targeting IGF-1 and IGF-2
Bispecific antibodies targeting IGF-1 and IGF-2 are designed to simultaneously block two important growth factor signaling pathways, thereby achieving a dual strike against cancer cells. These signaling pathways include:
-
PI3K/AKT signaling pathway: This is a key signaling pathway involved in cell survival, proliferation, metabolism, migration, and angiogenesis. The PI3K/AKT signaling pathway is tightly regulated in normal cells but is often activated or mutated in cancer cells, leading to tumor initiation, progression, and drug resistance. IGF-1 and IGF-2 bind to IGF-1R or IR, activating the PI3K/AKT signaling pathway, thereby promoting cancer cell survival and proliferation. Bispecific antibodies targeting IGF-1 and IGF-2 can inhibit the activation of this signaling pathway, thereby inducing cancer cell apoptosis or enhancing the effect of other therapies.
-
MAPK/ERK signaling pathway: This is an important signaling pathway involved in cell proliferation, differentiation, migration, invasion, and angiogenesis. The MAPK/ERK signaling pathway is regulated by various factors in normal cells but is often activated or mutated in cancer cells, leading to tumor initiation, progression, and metastasis. IGF-1 and IGF-2 bind to IGF-1R or IR, activating the MAPK/ERK signaling pathway, thereby promoting cancer cell proliferation and migration. Bispecific antibodies targeting IGF-1 and IGF-2 can inhibit the activation of this signaling pathway, thereby inhibiting cancer cell proliferation and migration.
-
Other signaling pathways: In addition to PI3K/AKT and MAPK/ERK signaling pathways, IGF-1 and IGF-2 can also affect the activity of other signaling pathways by binding to other receptors or binding proteins. For example, IGF-2 can bind to M6P/IGF-2R, affecting the TGF-beta signaling pathway; IGF-1 can bind to IGFBP-3, affecting the NFKB signaling pathway; IGF-1 and IGF-2 can also form hybrid receptors with ErbB family receptors or other growth factor receptors, affecting EGFR or HER2 signaling pathways. Bispecific antibodies targeting IGF-1 and IGF-2 can interfere with these signaling pathways interactions, thereby achieving multiple regulation of cancer cells.
Clinical Status of Bispecific Antibodies Targeting IGF-1 and IGF-2
Currently, there are no bispecific antibodies targeting IGF-1 and IGF-2 that have been approved for marketing. However, some bispecific antibodies targeting IGF-1R or IR and other targets have shown efficacy in clinical trials. For example, Xentuzumab (BI 836845) is a bispecific antibody that simultaneously targets IGF-1 and IGF-2, aiming to block their binding to IGF-1R and IR, thereby inhibiting their signaling pathways. BI 836845 is in phase II/III clinical trial in combination with everolimus for the treatment of hormone receptor-positive, HER2-negative breast cancer. LY3127804 is a bispecific antibody that targets IGF-1 and IGF-2, two ligands that bind to IGF-1R and insulin receptor and activate the signaling pathways that promote tumor growth and survival. It is currently in phase I/II clinical trial for patients with various solid tumors who have failed prior therapy.
Table 1. Example of Bispecific Antibodies Targeting IGF-1 and IGF-2
|
Bispecific Antibody
|
Developer
|
Clinical Trial Phase
|
Indication
|
Mechanism
|
|
Xentuzumab (BI 836845)
|
Boehringer Ingelheim
|
II/III
|
Breast cancer, pancreatic cancer, lung cancer, etc.
|
Blocking the binding of IGF-1 and IGF-2 to their receptors
|
The development of bispecific antibodies targeting IGF-1 and IGF-2 faces some challenges, such as the complexity of the IGF system, the heterogeneity of tumor expression and response, the potential toxicity and immunogenicity, and the optimal dose and schedule. Therefore, more studies are needed to optimize the design, selection, and combination of these agents, as well as to identify the most suitable patient population and biomarkers for their use.
References
1. Gkioka E, et al. Review: The Role of Insulin-like Growth Factor-1 Signaling Pathways in Uterine Leiomyoma. In Vivo. 2015 Nov-Dec;29(6):637-49.
2. Labrijn AF, et al. Therapeutic bispecific antibodies: the clinical landscape and challenges. Nat Rev Drug Discov. 2020 Nov;19(11):761-784.
3. Kontermann RE, et al. Bispecific antibodies. Drug Discov Today. 2015 Jun;20(7):838-847.
4. Spiess C, et al. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol. 2015 Oct;67(2 Pt A):95-106.
5. Brinkmann U, et al. Bispecific antibodies for cancer immunotherapy: current perspectives. BioDrugs. 2015 Apr;29(2):75-93.
6. Johnson S, et al. Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling. J Clin Immunol. 2010 Oct;30(5):737-750.
7. Xie Y, et al. Bispecific antibodies in cancer immunotherapy: mechanisms of action and clinical application strategies. Front Immunol. 2020 Nov 18;11:602016.
8. Fan G, et al. Bispecific antibodies and their applications in tumor immunotherapy. Signal Transduct Target Ther. 2019 Dec 20;4:62.
9. Wu J, et al. Advances in bispecific antibodies engineering: novel concepts for immunotherapies. Front Immunol. 2019 May 10;10:1047.
10. Stadler CR, et al. Eliminating toxicity while preserving antitumor activity of a bispecific antibody targeting IGF1R and EGFR in a mouse xenograft model of human pancreatic cancer cells with acquired resistance to cetuximab and erlotinib combination therapy. Mol Cancer Ther. 2014 Sep;13(9):2197-2208.
11. Wang Z, et al. A novel bispecific antibody targeting IGF1R and EGFR overcomes resistance to EGFR inhibitors in lung cancer cells with IGF1R activation or T790M mutation of EGFR gene. Oncotargets Therapies. 2020 Nov 23;13:12179-12191.
12. Wang S, et al. The state of the art of bispecific antibodies for treating human malignancies. EMBO Mol Med. 2021 Aug 9:e14291.
13. Ma J, et al. Bispecific Antibodies: From Research to Clinical Application. Front Immunol. 2021 May 5;12:626616.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY










