Introduction of CD3
CD3 is a complex of four distinct chains (γ, δ, ε, and ζ) that associate with the T cell receptor (TCR) and mediate T cell activation and signaling. CD3 is expressed on the surface of all T cells, regardless of their antigen specificity, and is encoded by genes located on chromosome 11 in humans. CD3 plays a crucial role in T cell development, differentiation, and function, as well as in various immune disorders such as autoimmune diseases, immunodeficiencies, and cancers. CD3 is a well-established target for bispecific antibodies (BsAbs) that aim to redirect T cells to tumor cells expressing a specific antigen, thereby inducing cytotoxicity and cytokine release.
Introduction of PMEL
PMEL is a type I transmembrane glycoprotein that is involved in melanosome biogenesis and pigmentation. PMEL is also known as SILV, gp100, or Pmel17, and is encoded by the PMEL gene located on chromosome 12 in humans. PMEL plays a key role in melanocyte differentiation, melanin synthesis, and melanoma progression, as well as in its immunogenicity and antigenicity in melanoma patients. PMEL is a promising target for bispecific antibodies (BsAbs) that aim to enhance the recognition and elimination of melanoma cells by T cells, especially in combination with other immunomodulatory agents.
Signaling Pathways Involved in Bispecific Antibodies Targeting CD3 and PMEL
The general mechanism of action of BsAbs targeting CD3 and PMEL involves the simultaneous binding of one arm to CD3 on T cells and the other arm to PMEL on melanoma cells, resulting in the formation of an immunological synapse and the activation of TCR signaling pathways. The main signaling pathways that are triggered by BsAbs targeting CD3 and PMEL include the MAPK/ERK, PI3K/AKT, NF-κB, and JAK/STAT pathways, which regulate various aspects of T cell function, such as proliferation, survival, differentiation, effector activity, and memory formation. These signaling pathways can modulate the anti-tumor efficacy and toxicity of BsAbs targeting CD3 and PMEL, as well as the potential strategies to optimize their therapeutic outcomes, such as using different formats, dosages, schedules, or combinations of BsAbs.
Clinic Status of Bispecific Antibodies Targeting CD3 and PMEL
Currently, the FDA has approved only one BsAb targeting CD3 and PMEL, tebentafusp (Immunocore's Kimmtrak®). This soluble TCR-based bispecific molecule received accelerated approval in May 2021 for treating patients with unresectable or metastatic uveal melanoma. Tebentafusp demonstrated significant overall survival improvement in a phase III trial (IMCgp100-202) compared to investigator's choice of therapy. Previous phase I and II trials (IMCgp100-101 and IMCgp100-102) also confirmed favorable safety, pharmacokinetics, pharmacodynamics, and biomarker profiles. Tebentafusp's mechanisms of action involve activating both CD8+ and CD4+ T cells, inducing a pro-inflammatory cytokine milieu, modulating the tumor microenvironment, and generating T cell memory.
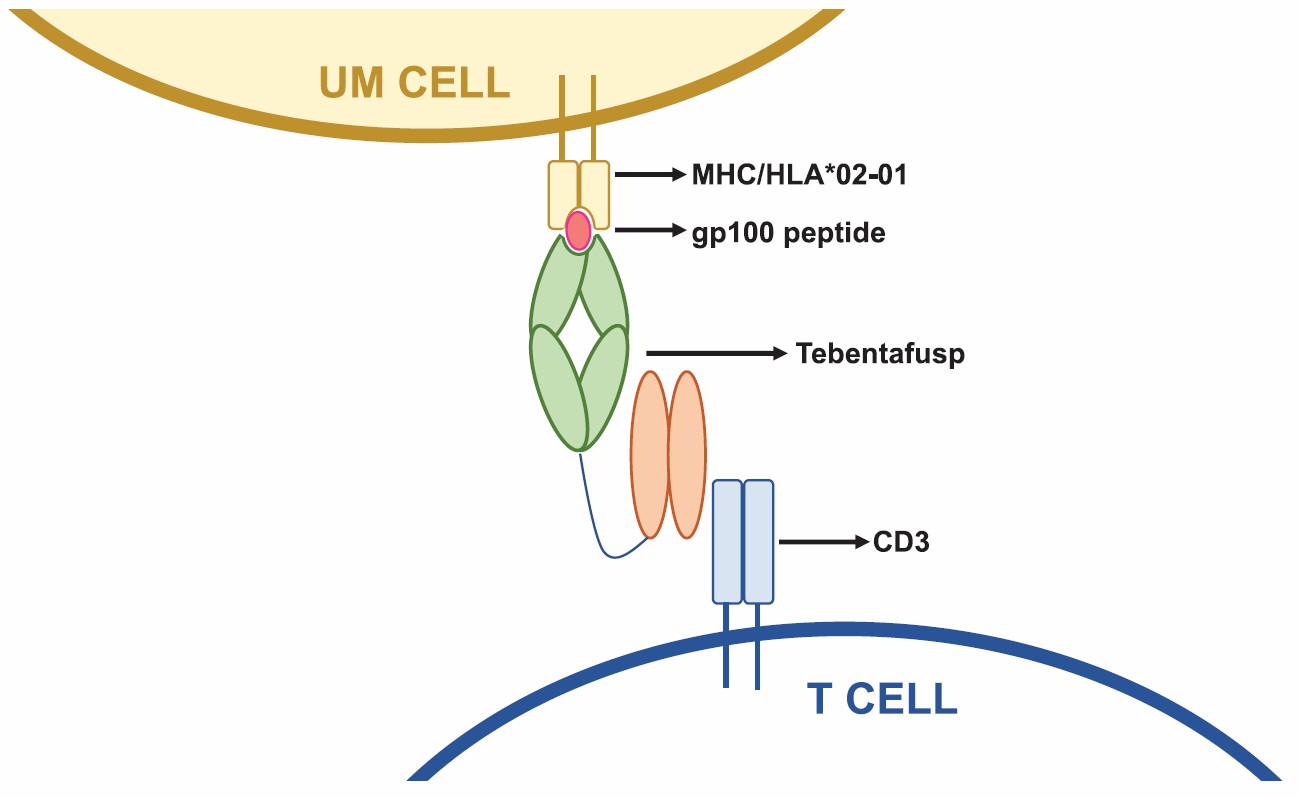
Fig.1 Schematic demonstrating the mechanism of action of tebentafusp (Howlett S, 2023)
Table 1. Summary of Tebentafusp
|
Name
|
Format
|
Developer
|
Target
|
Status
|
|
Tebentafusp
|
Soluble TCR-based bispecific molecule
|
Immunocore
|
CD3 and PMEL
|
Approved by FDA for metastatic uveal melanoma in May 2021 and of HLA-A*02:01-positive unresectable or metastatic ocular melanoma in January 2022
|
References
1. Crawford A, Chiu D. Targeting Solid Tumors Using CD3 Bispecific Antibodies. Mol Cancer Ther. 2021 Aug;20(8):1350-13581
2. Wei J, et al. Current landscape and future directions of bispecific antibodies in cancer immunotherapy. Front Immunol. 2022 Jan 28;13:10352762
3. Kontermann RE, Brinkmann U. Bispecific antibodies in oncology. Nat Rev Drug Discov. 2022 Feb;21(2):139-1603
4. Dheilly E, et al. Tebentafusp: T Cell Redirection for the Treatment of Metastatic Uveal Melanoma. Cancers (Basel). 2019 Jul 10;11(7):952.
5. Patel SP, et al. Tebentafusp induces antitumor immunity and clinical responses in patients with metastatic uveal melanoma: a phase I trial update with long-term follow-up. J Immunother Cancer. 2021 Feb;9(2):e001713.
6. Carvajal RD, et al. Effect of Tebentafusp vs Investigator’s Choice of Therapy on Overall Survival in Patients With Metastatic Uveal Melanoma: A Randomized Clinical Trial. JAMA Oncol. 2021 Sep 1;7(9):1315-1323.
7. Chen LN, Carvajal RD. Tebentafusp for the treatment of HLA-A*02:01-positive adult patients with unresectable or metastatic uveal melanoma. Expert Rev Anticancer Ther. 2022 Oct;22(10):1017-1027.
8. Nathan P, et al. Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. N Engl J Med. 2021 Sep 23;385(13):1196-1206.
9. Dhillon S. Tebentafusp: First Approval. Drugs. 2022 Apr;82(6):703-710.
10. Damato BE, et al. Tebentafusp: T Cell Redirection for the Treatment of Metastatic Uveal Melanoma. Cancers (Basel). 2019 Jul 11;11(7):971.
11. Middleton MR, et al. Tebentafusp, A TCR/Anti-CD3 Bispecific Fusion Protein Targeting gp100, Potently Activated Antitumor Immune Responses in Patients with Metastatic Melanoma. Clin Cancer Res. 2020 Nov 15;26(22):5869-5878.
12. Howlett S, et al. Tebentafusp: a first-in-class treatment for metastatic uveal melanoma. Ther Adv Med Oncol. 2023 Mar 21;15:17588359231160140.
13. Boudousquie C, et al. Polyfunctional response by ImmTAC (IMCgp100) redirected CD8+ and CD4+ T cells. Immunology. 2017 Nov;152(3):425-438.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY










