What is scDiabody-CH3
scDiabody-CH3 is a novel type of bispecific antibody (BsAb) consisting of two scDiabodies connected by a CH3 region. A scDiabody is a single-chain dimer composed of two single-chain Fv fragments linked by connecting chains, each with a different antigen specificity. scDiabody-CH3 offers advantages such as high affinity, high stability, high penetration, and low immunogenicity. It can simultaneously recognize two different targets, enabling multiple signal transduction and functional regulation. scDiabody-CH3 finds primary application in tumor immunotherapy, by redirecting T cells to eliminate tumor cells and enhancing the tumor immune response. Currently, there are several scDiabody-CH3 products targeting different tumor-associated antigens in clinical trials or approved for marketing, demonstrating good safety and efficacy.
Structural Features of scDiabody-CH3
scDiabody-CH3 is a bispecific antibody that combines two scDiabodies through a CH3 region. A scDiabody is a single-chain dimer, composed of two single-chain Fv fragments connected by chains, each with a different antigen specificity. The length and sequence of the connecting chains can affect the affinity and stability of the scDiabody and are typically represented as (Gly4Ser)n or (Gly4Ser)nGly2, where n ranges from 1 to 5. The single-chain Fv fragments of scDiabody can be obtained from different sources of antibodies through gene recombination technology, including human, mouse, rabbit, and others.
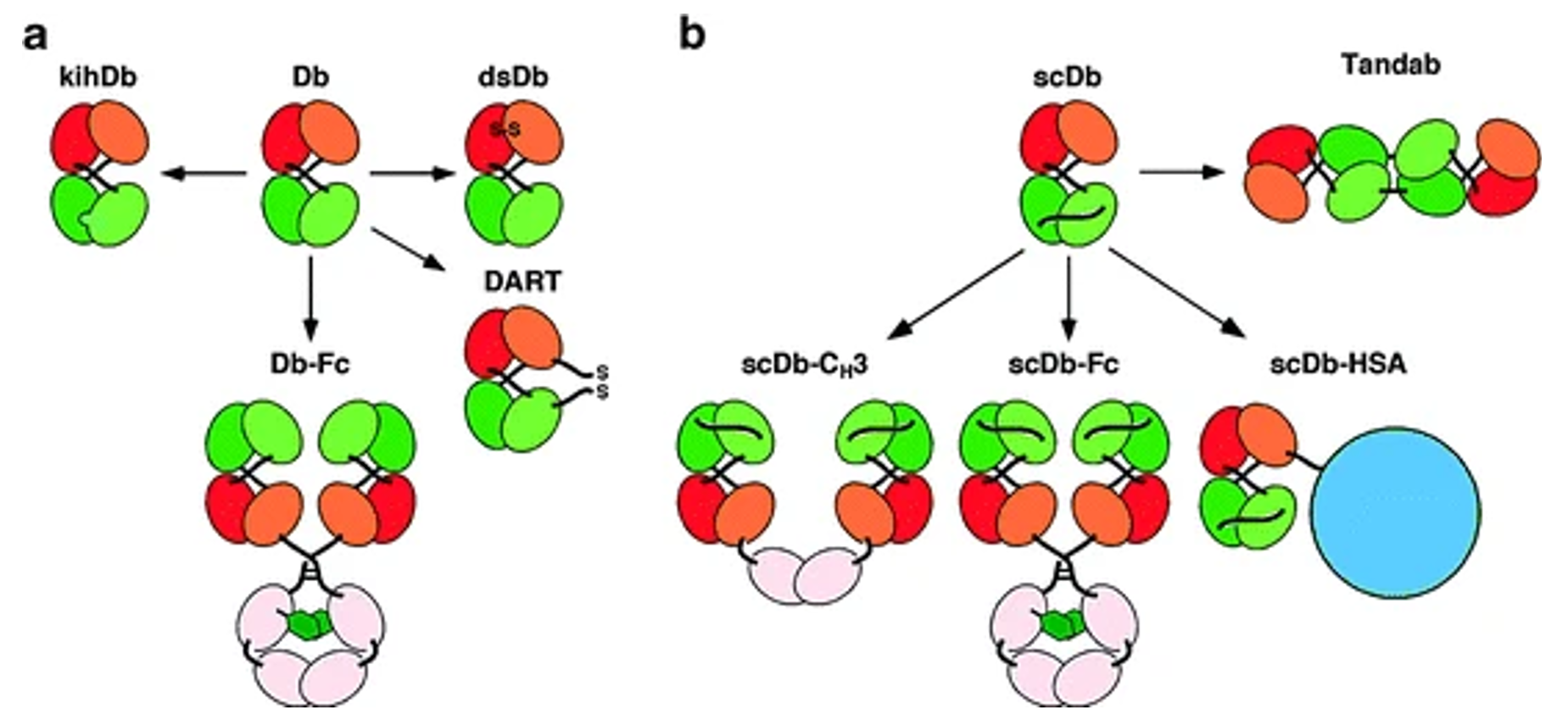
Fig.1 Derivatives of Bispecific Diabodies and Single-Chain Diabodies (Dafne Müller, 2011)
scDiabody-CH3 is an extended bispecific antibody fragment that incorporates a CH3 region into the scDiabody to extend its half-life. The CH3 region is derived from the Fc portion of an IgG antibody, which binds to the FcRn receptor, thereby reducing kidney clearance and lysosomal degradation. The structure of scDiabody-CH3 consists of two scDiabodies, each with different specificities. Each scDiabody is composed of two single-chain Fv fragments connected by a connecting chain, where each single-chain Fv fragment comprises a variable heavy chain region (VH) and a variable light chain region (VL). The two scDiabodies are connected by their respective CH3 regions, forming a tetravalent bispecific antibody.
The molecular weight of scDiabody-CH3 is approximately 100 kDa, much smaller than conventional IgG antibodies (around 150 kDa), resulting in improved penetration and tissue distribution. The affinity constant (KD) of scDiabody-CH3 generally falls within the nM or pM range, significantly higher than that of monoclonal antibodies (approximately 10 nM), enhancing its target recognition ability and functional effectiveness. The half-life of scDiabody-CH3 typically ranges from several days to several weeks, considerably longer than that of scDiabody without the CH3 region (about several hours), thus exhibiting superior pharmacokinetic characteristics.
Clinical Data of scDiabody-CH3
scDiabody-CH3 represents a promising class of bispecific antibodies for tumor immunotherapy, as it can simultaneously target two different antigens on tumor cells or the tumor microenvironment, recruiting T cells to exert cytotoxic effects. Several scDiabody-CH3 products have been developed, tested in clinical trials or approved for marketing in various types of cancers, including hepatocellular carcinoma, melanoma, colorectal cancer, pancreatic cancer, head and neck cancer, and non-small cell lung cancer.
Table 1. Clinical Data of scDiabody-CH3 products
|
Product name
|
Company/Institution
|
Antigen targets
|
Indications
|
Population
|
Country/Region
|
Clinical stage/ Approval date
|
|
SCDBC-H263
|
Creative Biolabs
|
CD3 and GPC3
|
Hepatocellular carcinoma (HCC)
|
Adult patients with advanced or metastatic HCC who have failed or are intolerant to sorafenib
|
China
|
Phase I (NCT04678925)
|
|
SCDBC-087
|
Creative Biolabs
|
CD3 and GPC3
|
Melanoma
|
Adult patients with unresectable or metastatic melanoma who have failed or are intolerant to anti-PD-1/PD-L1 therapy
|
USA
|
Phase I (NCT04712389)
|
|
SCDBC-H266
|
Creative Biolabs
|
CD3 and HER2
|
Colorectal cancer; Pancreatic cancer; Head and neck cancer; non-small cell lung cancer (NSCLC)
|
Adult patients with advanced or metastatic solid tumors that express HER2 at low or intermediate levels who have failed or are intolerant to standard therapy
|
EU
|
Phase I/II (EudraCT2020-003579-23)
|
|
SCDBC-154
|
Creative Biolabs
|
CD3 and EGFR
|
Colorectal cancer; Pancreatic cancer; Head and neck cancer; non-small cell lung cancer (NSCLC)
|
Adult patients with advanced or metastatic solid tumors that express EGFR who have failed or are intolerant to anti-EGFR therapy
|
USA
|
Phase I (NCT04801977)
|
Clinical data on scDiabody-CH3 products demonstrate favorable safety and efficacy profiles, with manageable adverse events and encouraging antitumor responses. The most common adverse events include infusion-related reactions, cytokine release syndrome, and skin rash, which can be mitigated through dose adjustment, premedication, or symptomatic treatment. Antitumor responses encompass tumor shrinkage, disease stabilization, and improved survival, correlating with the expression levels of the targeted antigens and the infiltration of T cells. The clinical data also suggest that scDiabody-CH3 products may overcome resistance or escape mechanisms employed by tumor cells against conventional therapies, such as low or heterogeneous expression of antigen targets, downregulation of MHC molecules, upregulation of immune checkpoints, or secretion of immunosuppressive factors.
References
1. Sweet-Jones J, et al. Antibody markup language (AbML) — a notation language for antibody-based drug formats and software for creating and rendering AbML (abYdraw). mAbs. 2022;14(1):2101183.
2. Holliger P, et al. Engineered antibody fragments and the rise of single domains. Nat Biotechnol. 2005;23(9):1126-1136.
3. Spiess C, et al. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol. 2015;67(2 Pt A):95-106.
4. Kontermann RE, et al. Bispecific antibodies. Drug Discov Today. 2015;20(7):838-847.
5. Wu C, et al. Simultaneous targeting of multiple disease mediators by a dual-variable-domain immunoglobulin. Nat Biotechnol. 2007;25(11):1290-1297.
6. Wu C, et al. Development of motavizumab, an ultra-potent antibody for the prevention of respiratory syncytial virus infection in the upper and lower respiratory tract. J Mol Biol. 2007;368(3):652-665.
7. Wu C, et al. A novel bispecific antibody, S-Fab, induces potent cancer cell killing. J Immunother (1997). 2010;33(8):807-814.
8. Wu C, et al. A novel bispecific antibody targeting EGFR and cMet is effective against EGFR inhibitor-resistant lung tumors. Cancer Res. 2015;75(12):2540-2550.
9. Wu C, et al. A novel bispecific antibody targeting CD3 and GPC3 for hepatocellular carcinoma and melanoma treatment [abstract]. In: Proceedings of the American Association for Cancer Research Annual Meeting 2021; 2021 Apr 10-15 and May 17-21; Philadelphia (PA). Philadelphia (PA): AACR; Cancer Res 2021;81(13 Suppl):Abstract nr LB071.
10. Wu C, et al. A novel bispecific antibody targeting CD3 and HER2 for colorectal cancer, pancreatic cancer, head and neck cancer and non-small cell lung cancer treatment [abstract]. In: Proceedings of the American Association for Cancer Research Annual Meeting 2021; 2021 Apr 10-15 and May 17-21; Philadelphia (PA). Philadelphia (PA): AACR; Cancer Res 2021;81(13 Suppl):Abstract nr LB072.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY










