What is Miniantibody
Miniantibodies are a class of small molecule antibodies that have high specificity and affinity, and can recognize and bind to the epitopes of antigens. Miniantibodies have two main sources: one is to discover and extract them from naturally existing small molecule antibodies, such as heavy chain antibodies from animals such as sharks, camels, llamas, etc.; the other is to construct and express them artificially by genetic engineering techniques, such as single-chain variable fragments (scFv), Fab fragments, single-domain antibodies (sdAbs), etc. Miniantibodies are classified mainly according to their structure, size, origin and function, such as monovalent, bivalent, multivalent, monospecific, bispecific, multispecific, etc. Miniantibodies have a wide range of applications, including biomedicine, diagnostics, therapeutics, biotechnology, such as detecting and neutralizing viruses, targeting tumor cells, modulating immune responses, and improving drug delivery. Miniantibodies have many advantages over traditional full-length antibodies, such as small molecular weight, high penetration, low immunogenicity, easy to modify and express, etc.
Structural Features of Miniantibodies
Miniantibodies have various structural features that determine their size, stability, affinity and valency. The basic structure of miniantibodies consists of a single variable domain (VH or VL) or a single-chain variable fragment (scFv) that contains both VH and VL domains connected by a flexible linker. The size of miniantibodies ranges from 12 to 30 kDa, which is much smaller than the conventional antibodies (150 kDa). The stability of miniantibodies depends on several factors, such as the amino acid sequence, the disulfide bonds, the glycosylation pattern, the folding pathway and the environmental conditions. Some miniantibodies have high thermal stability and resistance to pH changes, proteases and denaturants, while others may require engineering or fusion to stabilizing domains to improve their stability. The affinity of miniantibodies is mainly determined by the complementarity determining regions (CDRs) that interact with the antigen epitopes. The affinity of miniantibodies can be modulated by mutagenesis, selection or optimization of CDRs or framework regions. The valency of miniantibodies refers to the number of binding sites for the same or different antigens. The valency of miniantibodies can be increased by fusion to multimerization domains, such as Fc domains, helical domains or coiled-coil domains, resulting in bivalent, tetravalent or higher-order multivalent miniantibodies. The increased valency can enhance the avidity and functionality of miniantibodies, especially for low-affinity or low-density antigens.
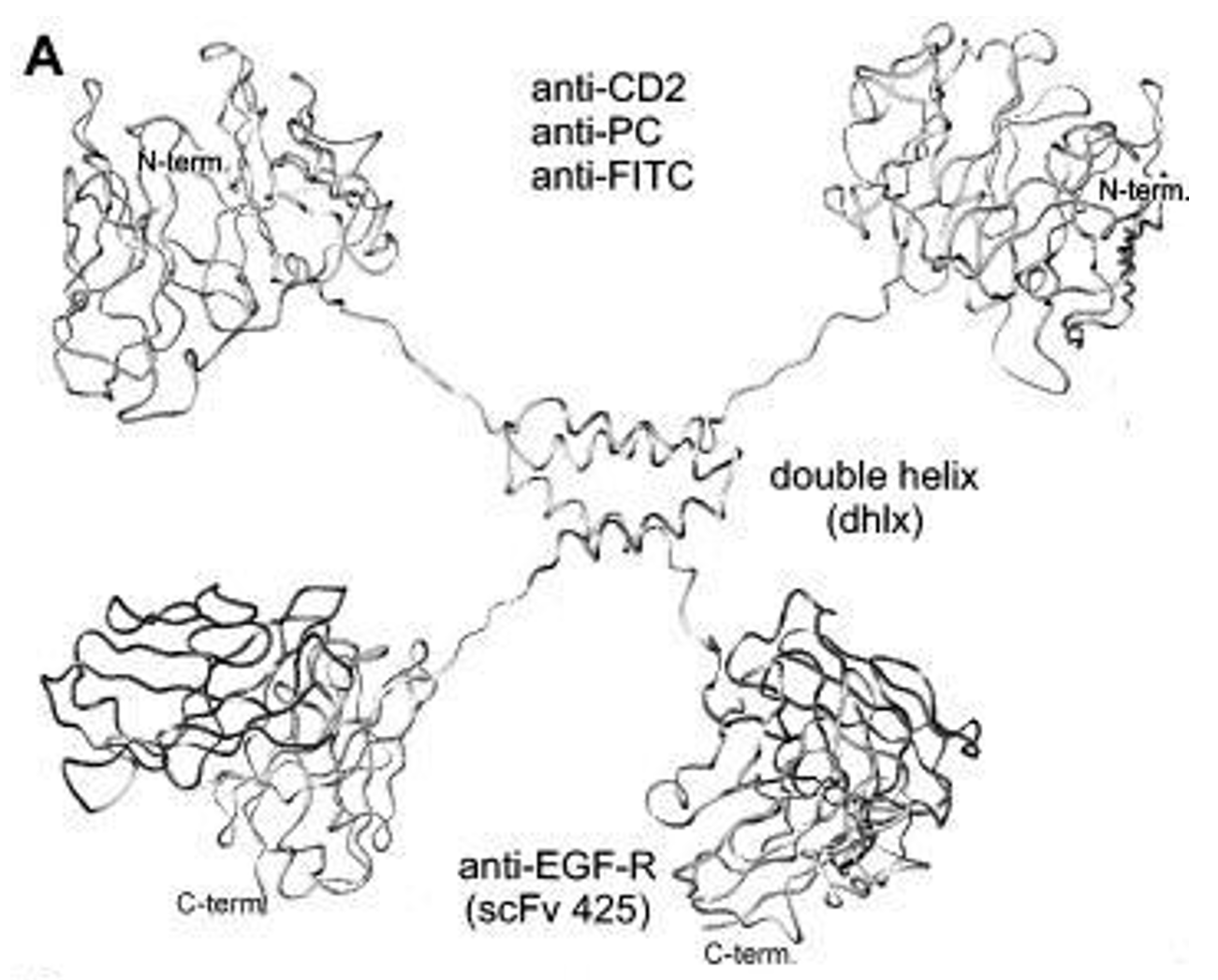
Fig.1 Model of a dimeric bispecific (DiBi) miniantibody. (Müller KM, 1998)
Generation Methods of Miniantibodies
Miniantibodies can be generated by various methods that involve either natural or artificial sources. The main generation methods are: Recombinant technology: This method involves cloning and expressing the genes encoding the variable domains of miniantibodies in suitable host cells, such as bacteria, yeast, insect or mammalian cells. Phage display technology: This method involves displaying the miniantibodies on the surface of bacteriophages and selecting them by binding to the target antigens. Transgenic animal technology: This method involves generating transgenic animals that carry human antibody genes and immunizing them with the target antigens.
Clinical Data of Miniantibodies
Miniantibodies have shown promising results in clinical trials and approvals for various diseases and targets. So far, only a few miniantibodies have been approved by the FDA or EMA for therapeutic use. These include dostarlimab, a monoclonal antibody that targets PD1 for the treatment of endometrial cancer; loncastuximab tesirine, an antibody-drug conjugate (ADC) that targets CD19 for the treatment of B-cell lymphoma; and amivantamab, a bispecific antibody that targets EGFR and MET for the treatment of non-small cell lung cancer (NSCLC) with EGFR exon 20 mutations.
Table 1. Information about these approved miniantibodies
|
Name
|
Target
|
Approval date
|
Indication
|
Population
|
Region
|
|
Dostarlimab
|
PD1
|
April 2021
|
Endometrial cancer
|
Patients with dMMR/MSI-H endometrial cancer that progressed on or after prior platinum-based chemotherapy
|
US
|
|
Loncastuximab tesirine
|
CD19
|
April 2021
|
B-cell lymphoma
|
Patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy
|
US
|
|
Amivantamab
|
EGFR×METR bispecific antibody
|
May-21
|
Non-small cell lung cancer (NSCLC)
|
Patients with EGFR exon 20 insertion-mutated NSCLC whose disease has progressed on or after platinum-based chemotherapy
|
US
|
There are many miniantibodies in different stages of clinical development for various diseases and targets. Some of the most advanced and promising ones are viltolarsen, an exon 53-skipping antisense oligonucleotide (ASO) for the treatment of Duchenne muscular dystrophy (DMD); efgartigimod, an FcRn-targeting antibody fragment for the treatment of autoimmune diseases such as myasthenia gravis, immune thrombocytopenia and pemphigus vulgaris; ALX-0171, an RSV F protein-targeting single-domain antibody (sdAb) for the treatment of respiratory syncytial virus (RSV) infection in infants; VHH-72Fc, a SARS-CoV-2 RBD-targeting single-domain antibody (sdAb) for the prevention and treatment of COVID-19 infection; and ALX-148, a CD47-targeting fusion protein for the treatment of various cancers.
Table 2. Information about these miniantibodies in clinical trials
|
Name
|
Target
|
Trial progress
|
Trial result
|
|
Viltolarsen
|
Exon 53-skipping ASO
|
Approved in Japan and US, undergoing III/IV trials
|
Significantly improved dystrophin expression and motor function in DMD patients
|
|
Efgartigimod
|
FcRn
|
Undergoing III trials
|
Improved primary endpoints in patients with myasthenia gravis and immune thrombocytopenia
|
|
ALX-0171
|
RSV F protein
|
Undergoing IIb trial
|
Reduced viral load and clinical score in infants with RSV infection
|
|
VHH-72Fc
|
SARS-CoV-2 RBD
|
Undergoing I/II trials
|
Showed high safety and neutralizing activity in animal models
|
|
ALX-148
|
CD47
|
Undergoing I/II trials
|
Showed preliminary efficacy in combination with other drugs for various cancers
|
References
1. Leslie M. Mini-antibodies discovered in sharks and camels could lead to drugs for cancer and other diseases. Science. 2018 May 10;360(6389):eaat4611.
2. Müller KM, et al. A dimeric bispecific miniantibody combines two specificities with avidity. FEBS Lett. 1998 Jul 31;432(1-2):45-9.
3. Müller D, et al. Miniantibodies: engineering approaches and applications. Methods Mol Biol. 2014;1060:1-18.
4. Wesolowski J, et al. Single domain antibodies: promising experimental and therapeutic tools in infection and immunity. Med Microbiol Immunol. 2009 Aug;198(3):157-74.
5. Muyldermans S. Single-domain antibodies (sdAbs): natural single-domain antibodies. Annu Rev Biochem. 2013;82:775-97.
6. Harmsen MM, et al. Properties, production, and applications of camelid single-domain antibody fragments. Appl Microbiol Biotechnol. 2007 May;77(1):13-22.
7. Hamers-Casterman C, et al. Naturally occurring antibodies devoid of light chains. Nature. 1993 Jun 3;363(6428):446-8.
8. Arbabi-Ghahroudi M. Camelid single-domain antibodies: historical perspective and future outlook. Front Immunol. 2017 Nov 20;8:1589.
9. Vincke C, et al. General strategy to humanize a camelid single-domain antibody and identification of a universal humanized single-domain antibody (sdAb) scaffold. J Biol Chem. 2009 Feb 6;284(6):3273-84.
10. De Genst EJ, et al. Molecular basis for the preferential cleft recognition by dromedary heavy-chain antibodies. Proc Natl Acad Sci U S A. 2006 Mar 14;103(11):4586-91.
11. Di Niro R, et al., Construction of miniantibodies for the in vivo study of human autoimmune diseases in animal models BMC Biotechnol., 2007 Aug 1;7:46.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY










