Creative Biolabs offers several high-quality biotin labeling services for our global customers for biomedical research use. Based on a wealth of experience and advanced technology, we are capable of designing and manufacturing both custom and customized antibody conjugation projects in close cooperation with our clients. In particular, our skilled experts enable us to offer quick and optimized conjugate results with excellent quality.
Biotin, also known as vitamin B7, is a small biologically active molecule that acts as a coenzyme in living cells. It can be coupled to oligonucleotides at the terminal and internal position at multiple sites. Biotin labeling (also called biotinylation) is the process of covalently attaching biotin to a protein, nucleic acid, or other molecule, which has been widely used in numerous detection systems especially when the target of interest is expressed at low levels and cannot be detected by labeled antibodies alone. Generally, biotinylation is rapid, and specific and is unlikely to perturb the natural function of the molecule due to the small size of biotin. Most importantly, many biotin molecules can be conjugated to an antibody with extremely high affinity, fast-on-rate, and high specificity, which does not appear in the conjugation with streptavidin and avidin. Through this amplification step, proteins which are expressed at low levels are more likely to be detected.
 Fig.1 Biotin maleimide cysteine reaction. Distributed under CC BY-SA 3.0, from Wiki, without modification.
Fig.1 Biotin maleimide cysteine reaction. Distributed under CC BY-SA 3.0, from Wiki, without modification.
The biotin tag can be used in the technique of affinity chromatography in conjunction with a column attached to avidin (or streptavidin or neutravidin), the natural ligand for biotin. Tagging the protein with iminobiotin can be utilized to isolate the tagged protein since the biotin analog has significant binding to avidin/streptavidin at alkaline pH but loses affinity when the pH is lowered. As a result, an avidin/streptavidin column can release an iminobiotin-tagged functional protein by reducing the pH (to around pH 4).
Anti-biotin antibodies or avidin/streptavidin-tagged detection techniques such as enzyme reporters or fluorescence probes, which can be employed to detect the protein, may facilitate localization using fluorescence or electron microscopy, ELISA assays, ELISPOT assays, western blots, and other immunoanalytical approaches. It is vital to highlight that utilizing monovalent streptavidin for detection prevents aggregation or aggregation of biotinylated targets.
The non-covalent bond formed by biotin and avidin or streptavidin is nearly as strong as a covalent bond and has a higher binding affinity than most antigen and antibody interactions. Based on this highly strong binding, labeling proteins with biotin is a useful tool for applications such as affinity chromatography, which separates the biotinylated protein from a mixture of other proteins and biochemicals using immobilized avidin or streptavidin. Biotinylation of MHC molecules to generate MHC multimers has also been shown to be an efficient approach for detecting and separating antigen-specific T-cell populations.
Protein biotinylation in living cells is developed to explore protein-protein interactions and proximity. One of the interacting proteins is biotinylated, while the other is being detected using a streptavidin-conjugated detection method. This method is frequently employed in pull-down assays and proximity-dependent labeling procedures.
Having provided protein biotin labeling service for many years, Creative Biolabs is capable of offering a full range of custom biotinylation services for both academic and industrial customers. With experienced scientists, we can label any protein with biotin as our clients require. Our advanced protein biotinylation platform enables us to provide simple and efficient biotin labeling without altering the bioactivity of target proteins. What's more, we have developed multiple biotin labeling approaches to satisfy worldwide customer's requirements.
Creative Biolabs is committed to offering high-quality biotin labeling services for worldwide customers. With advanced technology and proficient scientists, we can assure you both biotinylation efficiency and protein activity preservation. Moreover, our biotin labeling service is reproducible and quality guaranteed. For more detailed information, please feel free to contact us and get a quote. We will get in touch with you within 24 hours and design an optimal method for your project.
1. Cell Type-Specific Biotin Labeling for Proteomic Differences Profiling
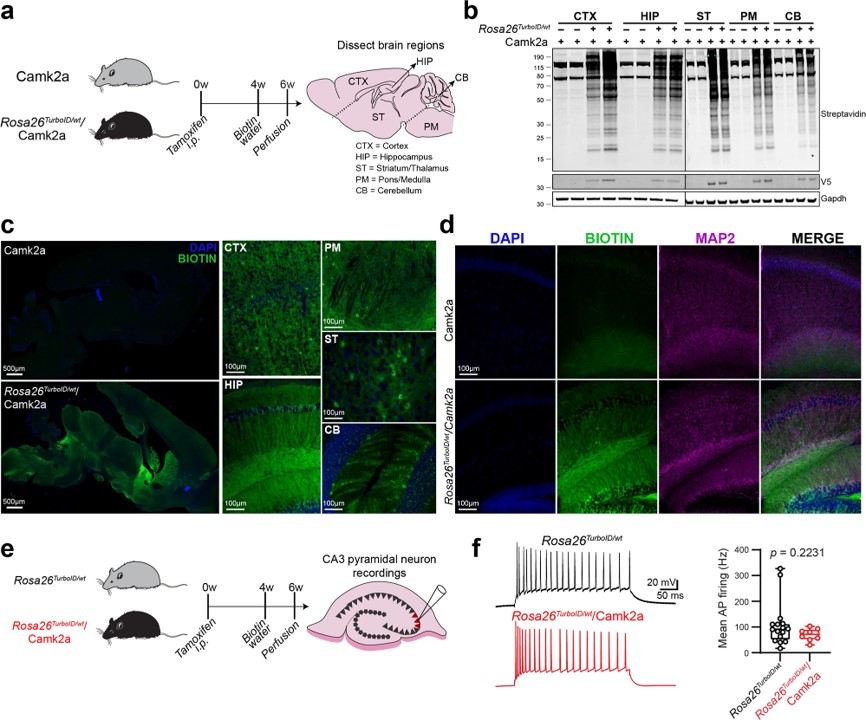 Fig.1 Biotin labeling of Camk2a-neuronal protein.1
Fig.1 Biotin labeling of Camk2a-neuronal protein.1
Researchers developed a mouse model for cell-type-specific expression of biotin ligase TurboID, which enabled in vivo protein biotinylation. Using adenoviral and transgenic methods, they achieved widespread protein biotinylation of neurons, labeling proteins across neuronal soma and axons all over the brain. This allowed the identification of over 2,000 neuron-derived proteins, including transporters, synaptic proteins, ion channels, and potential drug targets. The team compared the proteomes of Camk2a-neurons and Aldh1l1-astrocytes, revealing region-specific differences that may contribute to selective vulnerability to neurological diseases. Additionally, they applied an antibody-based technique to explore differences in signaling phospho-proteins and cytokines between neurons and astrocytes. This approach offers a powerful tool for studying cell-type-specific proteomes in various tissues under both normal and disease conditions.
2. Proximity-Dependent Biotin Labeling for Protein-Protein Interactions Characterization
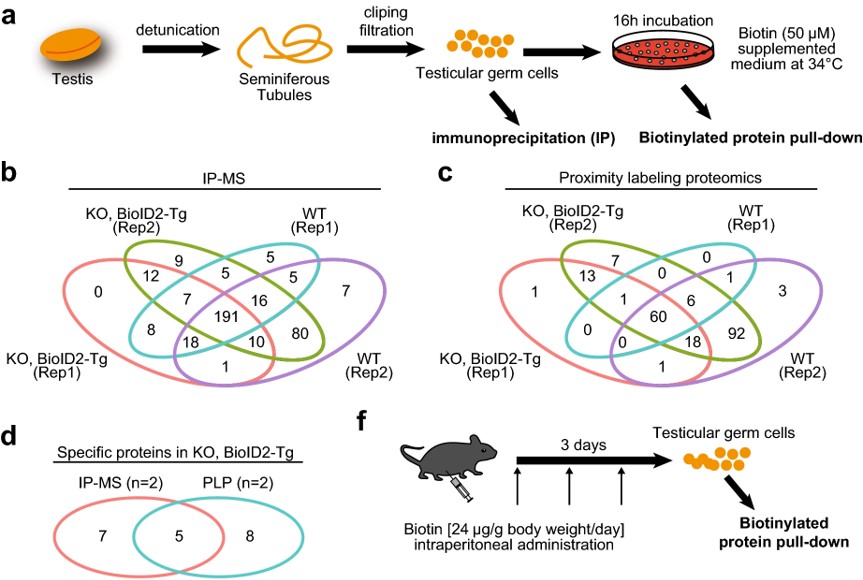 Fig.2 Interactome analysis of TESMIN.2
Fig.2 Interactome analysis of TESMIN.2
Proximity-dependent biotin identification (BioID) is a promising method for mapping transient protein-protein interactions. In this study, researchers applied proximity labeling proteomics to the testis by creating two transgenic mouse lines expressing biotin ligases (BioID2 or TurboID) fused with TESMIN, a protein that translocates from the cytosol to the nucleus during meiotic progression and is essential for reproduction. The BioID2 fusion rescued fertility defects in Tesmin KO male mice, demonstrating that TESMIN-BioID2 can functionally replace TESMIN. Biotinylated protein pull-down followed by mass spectrometry identified components of the MYBL1-MuvB complex, which regulate cell-cycle gene expression. This study highlights the utility of proximity labeling proteomics in fully identifying TESMIN-associated proteins in male germ cells.
References
For Research Use Only.
