Currently, invasive fungal infections are considered a severe threat to human health. Mortality rates from untreated systemic fungal infections normally exceed 98%. Despite the intensification therapy with existing antifungal agents, permanent cures are uncommon. These facts indicate that it is necessary to develop novel antifungal strategies constantly. Creative Biolabs now provides a comprehensive series of antifungal drug discovery services, including target identification and validation for homoisocitrate dehydrogenase as a potential target.
Homoisocitrate Dehydrogenase
Homoisocitrate dehydrogenase (HICDH), which is also known as 2-hydroxy-3-carboxyadipate dehydrogenase and 3-carboxy-2-hydroxyadipate dehydrogenase, is an enzyme that plays a role in lysine biosynthesis. HICDH belongs to the family of oxidoreductases, specifically those acting on the CH-OH group of donors with NAD+ or NADP+ as acceptor. It is a member of the family of pyridine nucleotide-dependent β-hydroxyacid oxidative decarboxylases. HICDH catalyzes the fourth reaction of the α-aminoadipate pathway, specifically, the NAD+-dependent conversion of homoisocitrate to α-ketoadipate (α-Ka).
Homoisocitrate Dehydrogenase as a Target for Antifungal Drug Discovery
The α-aminoadipate pathway (APP) is characterized by the initial condensation of α-ketoglutarate and acetyl-CoA, catalyzed by the homocitrate synthase, resulting in the generation of the first key intermediate, homocitrate. Homocitrate is converted into homoisocitrate by de- and rehydration reactions. The α-aminoadipate pathway is essential for lysine synthesis in all higher fungi. This pathway is proved to be found only in fungi, but not in plants or animals.
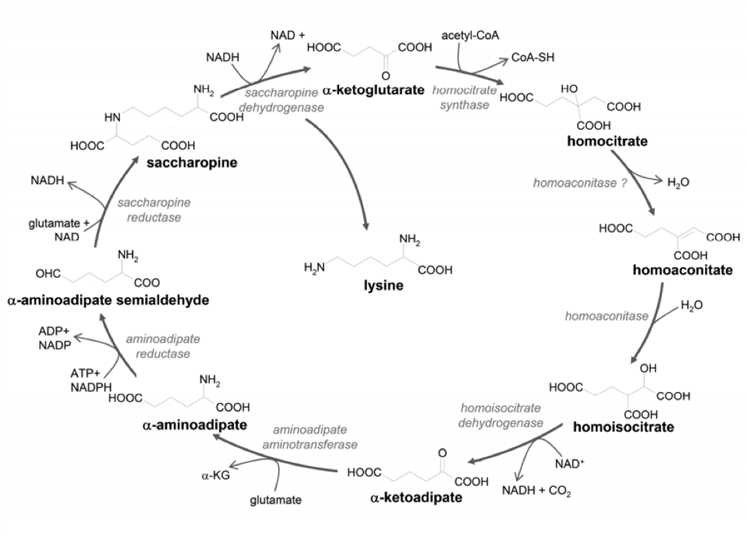 Fig.1 Scheme of the α-aminoadipate pathway. (Schöbel, 2010)
Fig.1 Scheme of the α-aminoadipate pathway. (Schöbel, 2010)
Therefore, this pathway could be regarded as a target for antifungal drugs since its disruption should not have an impact on the metabolism of host organisms. Since HICDH catalyzes biosynthetic reactions present only in fungi and having no counterparts in mammals, this enzyme is the most obvious candidates for the molecular targets. Moreover, compounds designed to mimic the structure of the metabolites of interest are proved to inhibit homoaconitase and/or homoisocitrate dehydrogenase, which leads to an impairment in lysine biosynthesis.
Why Choose Us?
The scientific group for drug development in Creative Biolabs consists of highly experienced leading experts that focused on antifungal studies. We aim to assess needs and advance to future technologies and new therapy developments. Aided by our fully-trained technical support team, we now provide the best strategy and protocols to launch any antifungal drug discovery project, especially for Fungal Nucleic Acid and Protein Biosynthesis Targets, including:
In addition to the identification of potential targets for antifungal drug discovery, you might be also interested in other services for Potential Targets for Antifungal Drug Discovery listed below:
If you have any special needs in discovering potential targets for antifungal drugs or be interested in learning more about our antifungal drug discovery services, please feel free to contact us for more details.
Reference
-
Schöbel, F.; et al. Evaluation of lysine biosynthesis as an antifungal drug target: biochemical characterization of Aspergillus fumigatus homocitrate synthase and virulence studies. Eukaryotic cell. 2010, 9(6), pp.878-893.
For Research Use Only.


 Fig.1 Scheme of the α-aminoadipate pathway. (Schöbel, 2010)
Fig.1 Scheme of the α-aminoadipate pathway. (Schöbel, 2010)


