Drug Metabolic Stability Analysis Service
In vitro metabolic stability assays are important in drug development as they help screen for therapeutic drugs with favorable metabolic properties. Therapeutic drugs that are rapidly metabolized in the body may not have enough time to exert their desired therapeutic effect, leading to lower bioavailability and decreased efficacy. Conversely, drugs that are highly stable and resistant to metabolism may persist in the body for too long, increasing the risk of adverse effects or toxicity. At Creative Biolabs, we offer comprehensive metabolic stability assay services to help identify drugs with optimal metabolic stability. Our metabolic stability assays are designed to mimic the physiological conditions of the human body, providing a reliable prediction of how a drug will behave in vivo.
Common Test Systems
To evaluate the drug's metabolic stability, several in vitro test systems are commonly used. These systems mimic the enzymatic environment found in the human body, allowing researchers to assess how compounds interact with and are metabolized by various enzymes. Common test systems include hepatocytes, microsomes, and cell-free systems (recombinant enzymes or enzyme mixtures). A combination of different test systems is often used to comprehensively evaluate the metabolic stability of drugs.
-
Microsomes
-
Hepatocytes
-
S9 fraction
-
Recombinant CYP enzymes
-
Plasma
Our Services
Metabolic Stability Assays
Metabolic stability assays are used to evaluate the rate at which a drug is metabolized or broken down in biological systems, typically in liver microsomes or hepatocytes which contain the enzymes responsible for the majority of drug metabolism in the body. We provide several different types of metabolic stability assays, including:
-
Microsome/S9 stability assays: These assays use liver microsomes or S9 fraction, which contain the enzymes responsible for drug metabolism, to evaluate the stability of a drug in vitro.
-
Hepatocyte stability assays: These assays use isolated hepatocytes to evaluate the stability of a compound in a more complex cellular environment.
-
Plasma stability assays: Plasma metabolic stability assessment involves evaluating the stability of a compound in plasma samples obtained from humans or animals.
-
Recombinant enzyme stability assays: A mixture of recombinant enzymes (e.g. CYP1A2, CYP2C9, CYP3A4, UGT1A1, UGT1A4, GSTM1, and MAOA) are used to mimic the metabolic activity that would occur in the body.
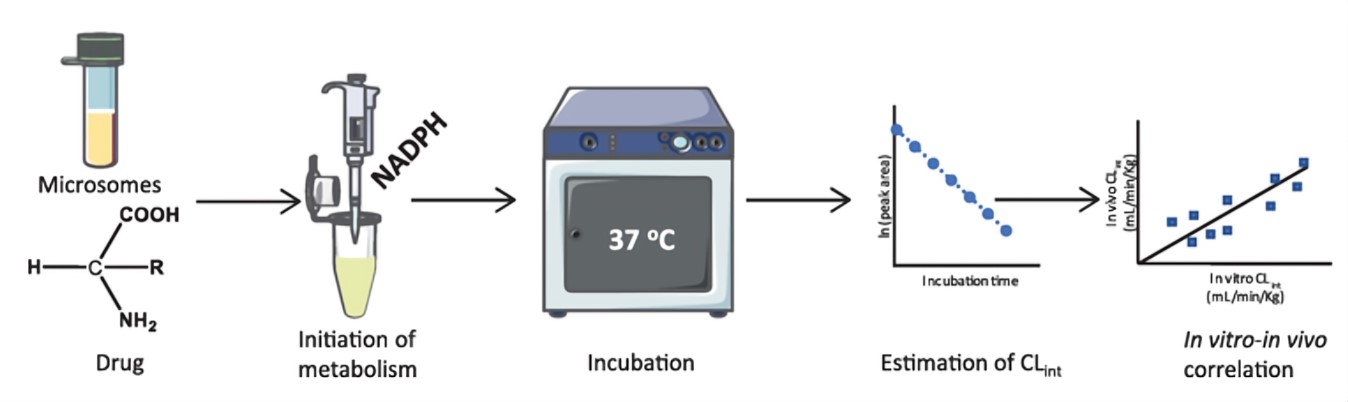 Fig.1 Drug metabolic stability analysis in a microsome system.1
Fig.1 Drug metabolic stability analysis in a microsome system.1
Metabolic Profiling Service
Additionally, metabolic profiling is also important in drug development. This involves the identification and quantification of metabolites formed during the metabolism of a drug. Metabolites can be analyzed using various techniques, such as LC-MS or NMR. Information obtained from metabolic profiling can be used to identify potential reactive intermediates or toxic metabolites that may contribute to adverse effects or toxicity observed in vivo.
At Creative Biolabs, we are committed to providing our clients with the highest quality data and insights to support their drug development efforts. Our metabolic stability assay services are tailored to meet the specific needs of each client, ensuring that they receive the most relevant and accurate information to guide their drug discovery and development process.
Reference
-
Gajula, Siva Nageswara Rao, et al. "In vitro drug metabolism studies using human liver microsomes." Dosage Forms-Innovation and Future Perspectives. IntechOpen, 2022.
For Research Use Only | Not For Clinical Use


 Fig.1 Drug metabolic stability analysis in a microsome system.1
Fig.1 Drug metabolic stability analysis in a microsome system.1
 Download our brochure
Download our brochure