Antibody-Drug Conjugate (ADC) Technology
 Fig.1 Antibody-drug conjugate.Antibody-drug conjugate (ADC) aims to selectively deliver cytotoxic drugs preferentially to antigen-expressing tumor cells utilizing the specificity of monoclonal antibodies (mAbs). In contrast to traditional cytotoxic drugs, ADC increases the efficacy and decreases the toxicity of its payloads. The targeted delivery of cytotoxic drugs to cancer cells raises the percentage of drug molecules reaching tumors, thereby lowering the minimum effective dose and elevating the maximum tolerated dose.
Fig.1 Antibody-drug conjugate.Antibody-drug conjugate (ADC) aims to selectively deliver cytotoxic drugs preferentially to antigen-expressing tumor cells utilizing the specificity of monoclonal antibodies (mAbs). In contrast to traditional cytotoxic drugs, ADC increases the efficacy and decreases the toxicity of its payloads. The targeted delivery of cytotoxic drugs to cancer cells raises the percentage of drug molecules reaching tumors, thereby lowering the minimum effective dose and elevating the maximum tolerated dose.
ADC represents an innovative therapeutic approach that offers the promise for increased drug specificity and fewer off-target effects than chemotherapy. Therefore, numerous new ADC medicines are in different stages of clinical development. ADC that have been approved by FDA includes brentuximab vedotin for CD30-positive Hodgkin's lymphoma and trastuzumab emtansine for human epidermal growth factor receptor 2-positive breast cancer. Creative Biolabs is at the leading edge of medicine development and ADC research. This novel technology to therapy provides meaningful efficacy while potentially limiting side effects.
Antibody-drug Conjugate (ADC) Technology at Creative Biolabs
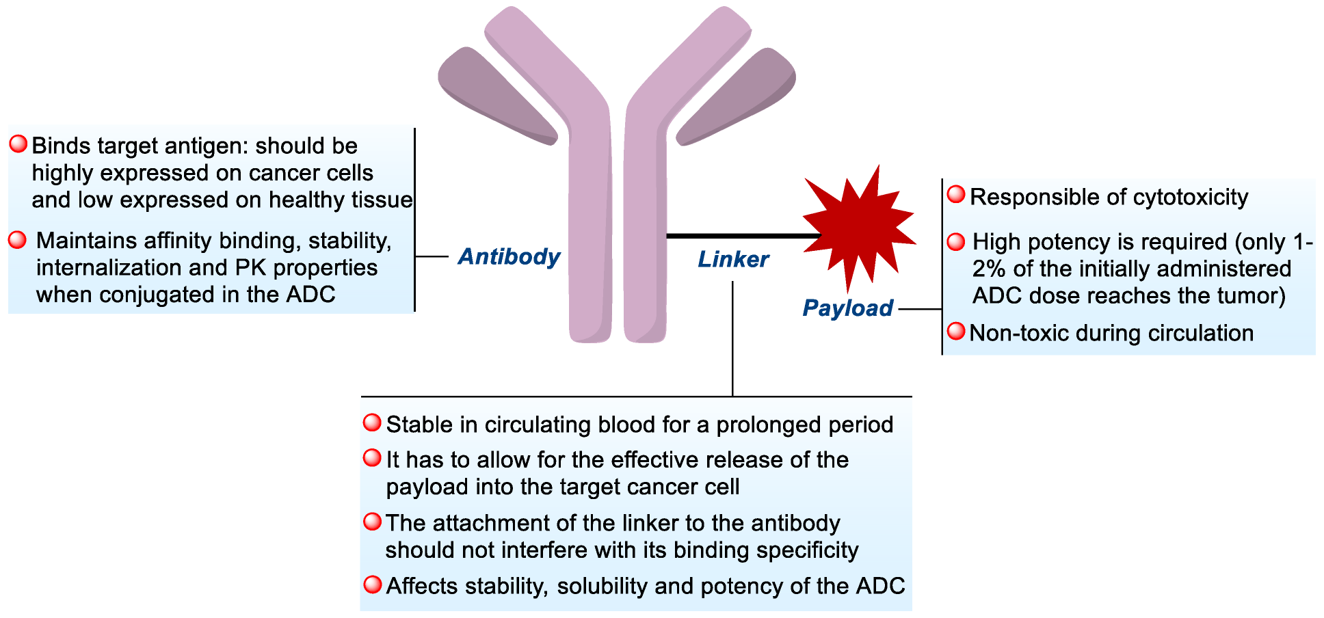 Fig.2 General structure of antibody-drug conjugate.1
Fig.2 General structure of antibody-drug conjugate.1
ADC is a complex immunoconjugate that consists of a monoclonal antibody directed to a tumor antigen and a cytotoxic agent conjugated to the antibody by a molecular linker. Although the concept is simple, the success of ADC depends on careful optimization of each building block, antibody, payload, and linker. Creative Biolabs has a comprehensive portfolio of products to meet ADC manufacturing needs and can provide dedicated equipment to characterize the purity, homogeneity, and stability of ADC.
➢ Monoclonal Antibody
Advances in antibody have enabled identification and generation of humanized or human antibodies with high-affinity, highly-selectivity for a target of interest. Today, the knowledge of disease biology and engineering antibodies with ideal properties become the foundation of our ADC. We have adopted a number of strategies in antibody design and engineering to create new monoclonal antibodies with suitable conjugation sites for the generation of ADC.
-
Selection and optimization of antibodies:
-
Antibody screening
-
Isotype selection
-
Chimeric antibodies
-
Humanized and human antibodies
Furthermore, the elements of ADC through our platform meet the following conditions. i) High specificity & binding affinity to target antigens on cells; ii) Efficient intracellular trafficking & long circulation time; iii) Low immunogenicity; iv) Effector function mediated antitumor activity towards target cells (e.g. ADCC or CDC); v) Retention of above features after conjugation.
➢ Cytotoxic Payload
The design of the cytotoxic payload is what makes the ADC a powerful therapeutic tool. The payload is an active substance that kills tumor cells typically through interference with cell mitotic cycles by binding to tubulin or damaging DNA via alkylation or cleavage. The current payloads engineered by us in the clinical development are all natural product-derived molecules with picomolar (pmol) or better potencies. There are several commonly used toxic payloads.
-
DNA binding cytotoxic agents - Calicheamicins, Duocarmycins
-
Tubulin inhibitors - Auristatins (monomethyl auristatin E, MMAE; monomethyl auristatin F, MMAF), Maytansinoids (DM1, DM4)
At Creative Biolabs, we are still focusing on the development of new cytotoxic agents that show higher potency, overcome drug resistance, and unknown mechanisms of action, in addition to our proven cytotoxic agents.
➢ Linker Design
The focus of ADC is the linker between the antibody and cytotoxic payload. The linker is bifunctional, conjugating the relatively small molecule payload to the larger biomolecule antibody by orthogonal chemistry. There, one functional group only reacts with the payload while the other group conjugates to the antibody. An ideal linker contains simple, cost-effective, high yield chemicals for attachment to the toxic payload (generally organic solvent based) and allowing gentle, aqueous-based bioconjugation to the antibody.
-
Novel conjugation strategies:
-
Conventional Conjugation - Native cysteine conjugation, Lysine conjugation;
-
Site-specific Conjugation - Engineered cysteine conjugation, Enzymatic conjugation, Unnatural amino acids as conjugation sites, Glycan conjugation;
-
Amino‑terminal engineered serine conjugation - The aldehydes reacted from serines are used for oxime ligation.
-
Fab nucleotide-binding site conjugation - By conjugated indole-based 5‑difluoro‑2,4‑dinitrobenzene linker.
-
Native cysteine rebridging - Using bis-alkylation conjugation at reduced interchain disulfides;
-
Avoiding retro-Michael deconjugation - Michael addition of a thiol to a maleimide used for bioconjugation of drugs to antibodies.
In Creative Biolabs, we insist on innovating new classes of linkers to specifically deliver the maximum cell-killing dose to target cells, improving antitumor activity while minimizing side effects for patients. Moreover, we have established a Pt-based conjugation technology, PtLnX, which utilizes bis(ethylenediamine) platinum chloride [Pt(en)Cl2] as a linker core for ADC development.
Next Generation ADC Technology
The convergence of in-depth understanding of biology with chemistry has led to encouraging progress in the development of ADC. The first generation of ADC is commercialized utilizing lysine and cysteine chemistry, and the second generation ADC involves specific site conjugation technology that is being tested, but with narrow windows because of off-target toxicity. Here, Creative Biolabs focuses on the third generation of ADC technologies to expand the treatment. The toxicity reported for active, discontinued medicines, the optimization of antibody, and the linker/conjugation chemistry are significant to drive the rational design and promote the therapeutic index of the third generation ADC.
Featured Services at Creative Biolabs
Creative Biolabs's ADC technology combines the specificity of antibodies, novel linker systems, and powerful cell-killing agents to fight cancers and improve the prognosis of patients. Through our proprietary methods, new linkers have been synthesized and highly potent cytotoxic payloads have been created for optimized ADC products to potentially offer treatment options at clinical stages. If there is any query, please contact us for more details.
Reference
-
Cheng-Sánchez, Iván, et al. "Antibody-drug conjugates containing payloads from marine origin." Marine Drugs 20.8 (2022): 494.
For Research Use Only | Not For Clinical Use
Related Services


 Fig.1 Antibody-drug conjugate.
Fig.1 Antibody-drug conjugate. Fig.2 General structure of antibody-drug conjugate.1
Fig.2 General structure of antibody-drug conjugate.1
 Download our brochure
Download our brochure