B Cell-based Calorimetry Assay
Principal of the Calorimetry Assay
In general, the interaction process between biomolecules often causes heat release or heat absorption. The isothermal titration calorimetry (ITC) assay is used to directly measure the amount of heat released or absorbed between protein and protein interactions in solution, which is convenient and widely applied to quantify the binding affinity.
The experiment is carried out in an isothermal titration calorimeter at a constant temperature. Constant power is delivered to the reference cell initially. After ligand loading, the binding between the ligand and the sample cell induced temperature change. The temperature needed to bring the sample cell to the same level as the reference cell is translated to power and then conversed to a binding enthalpy in molar terms.
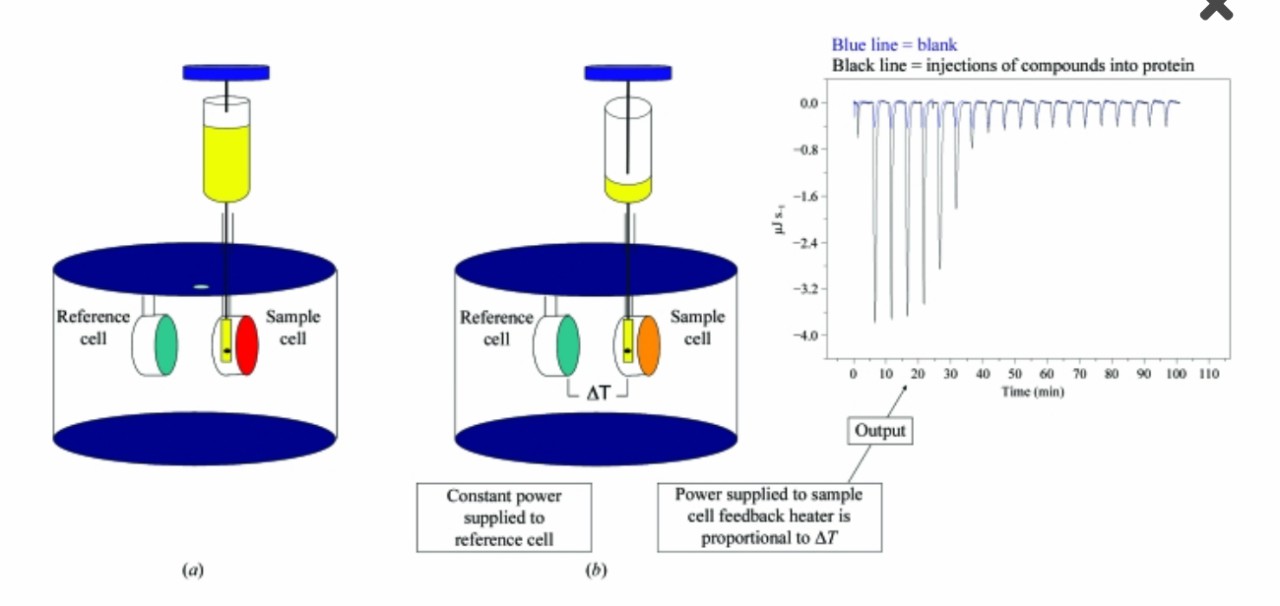 Fig.1 Schematic diagram of an isothermal titration calorimetry instrument.1
Fig.1 Schematic diagram of an isothermal titration calorimetry instrument.1
Calorimetry Assay Services at Creative Biolabs
ITC is the gold-standard method that directly measures the binding enthalpy and stoichiometry during antibody-antigen interactions. Creative Biolabs provides antibody-based calorimetry assay for cancer epitope studies to determine the binding kinetic parameters of specific antibody-epitope complexes and make a better understanding of tumor antigens.
Workflow

Animal Models Available
✔ Vicugna pacos
✔ Lama glama
✔ Mouse
✔ Camel
 Fig.2 Produce nanobody from lama glama.3
Fig.2 Produce nanobody from lama glama.3
Our Capabilities
Isothermal titration calorimetry (ITC) assay is used to measure antigen-antibody binding interaction qualitatively and quantitatively.
-
The change in enthalpy (ΔH) from the heat released or absorbed during the association reaction between antibody and epitope.
-
The association constant KA and dissociation constant KD. KD is more commonly used than KA and is reverse proportional to the binding affinity. A smaller KD value means a tight binding affinity.
-
Isothermal titrations of full-length antibody or corresponding divalent F(ab′)2 fragments or monovalent Fab fragments into purified antigen proteins.
-
Modify binding stoichiometries of antibodies providing the thermodynamic basis to understand the interaction.
Data Interpretation
The selective and specific binding between an epitope with an antibody is identified by comparing the KD value.
|
KD
|
Affinity
|
|
nM, 10-9 M level
|
Strong
|
|
μM, 10-6 M level
|
Medium-strength
|
|
mM, 10-3 M level
|
Weak
|
Key Features of Our ITC Assay
-
Our ITC assay is versatile and measures a wide range of biomolecules.
-
Samples are prepared in solutions and do not need any labeling.
-
The minimal reagent is necessary for developing an assay.
-
Directly measure binding affinity in sub-millimolar to nanomolar.
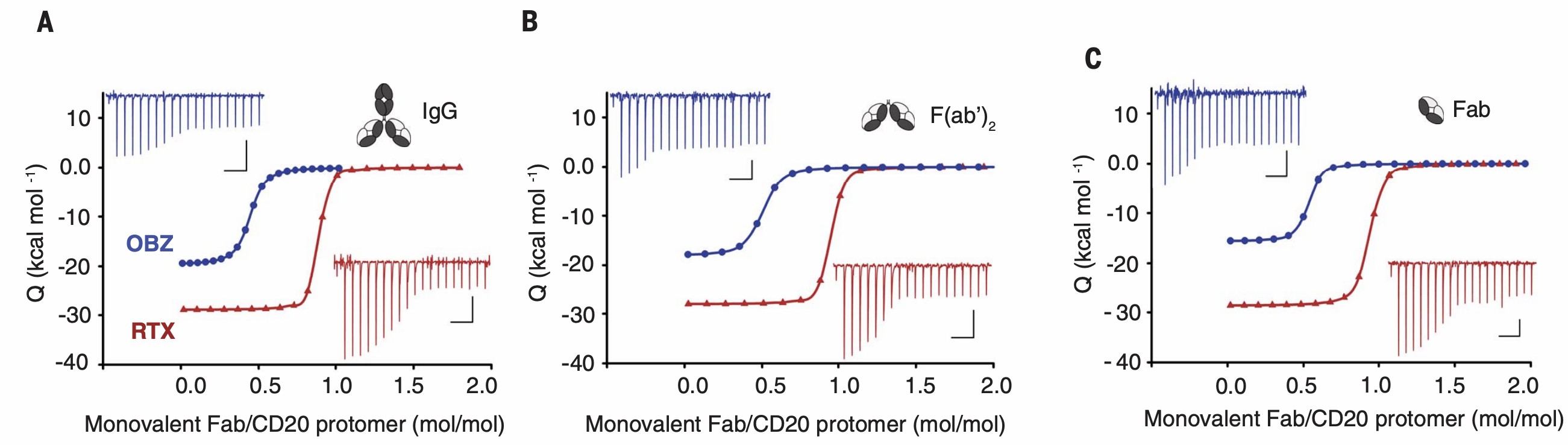 Fig.3 Results of isothermal titrations of IgG and variants F(ab')2 and Fab antibodies into CD20 proteins.2
Fig.3 Results of isothermal titrations of IgG and variants F(ab')2 and Fab antibodies into CD20 proteins.2
Related Services
Surface plasmon resonance is also a sensitive technique to detect antigen-antibody interaction and is recommended for positive antibody clones and epitope verification.
Creative Biolabs provides trusted services to accelerate your cancer research and drug development. We will work closely with our partner to outline your specific requirements and objectives and fulfill them on time. For further details, please don't hesitate to contact us.
References
-
Chung, Chun-wa. The use of biophysical methods increases success in obtaining liganded crystal structures. Acta crystallographica. Section D, Biological crystallography. 2007, 63: 62-71.
-
Kumar, A.; et al. Binding mechanisms of therapeutic antibodies to human CD20. Science (New York, N.Y.). 2020, 369(6505): 793–799.
-
Kim, H. J.; et al. Isolation of alpaca anti-hapten heavy chain single domain antibodies for development of sensitive immunoassay. Analytical chemistry. 2012, 84(2): 1165–1171.
For Research Use Only | Not For Clinical Use


 Fig.1 Schematic diagram of an isothermal titration calorimetry instrument.1
Fig.1 Schematic diagram of an isothermal titration calorimetry instrument.1

 Fig.2 Produce nanobody from lama glama.3
Fig.2 Produce nanobody from lama glama.3
 Fig.3 Results of isothermal titrations of IgG and variants F(ab')2 and Fab antibodies into CD20 proteins.2
Fig.3 Results of isothermal titrations of IgG and variants F(ab')2 and Fab antibodies into CD20 proteins.2
 Download our brochure
Download our brochure