Extraction Assay
Creative Biolabs provides extraction assays to remove endotoxin from cytochrome c, catalase, and serum proteins.
Our Services
The mechanism of solvent extraction is used to separate endotoxins from target therapeutics based on their relative solubilities in two immiscible liquids. As a result, endotoxin forms partition in the organic phase, while hydrophilic target molecules remain in the aqueous phase. Creative Biolabs offers different solvents to satisfy customers' needs in endotoxin removal projects.
-
Triton X-114
Detergent Triton X-114, a non-ionic surfactant, is a kind of two-phase extraction, which has been explored to remove endotoxins from target therapeutics. It has been reported that endotoxin is successfully removed from the green fluorescent protein using Triton X-114 and temperature transitions. Triton X-114 is characterized as miscible with water at a temperature of 0°C, but a phase separation occurs at temperatures above 23°C. Endotoxins are partitioned in the detergent phase, while target therapeutics are partitioned in the aqueous phase. In addition, Triton X-114 isothermal extraction using sodium dodecyl sulfate (SDS) has been very effective in removing endotoxins from pDNA.
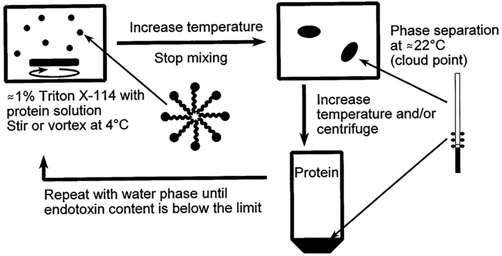 Fig.1 Removal of endotoxins by two-phase extraction with Triton X-114. (Petsch, 2000)
Fig.1 Removal of endotoxins by two-phase extraction with Triton X-114. (Petsch, 2000)
Features:
✔ Endotoxin removal efficiencies using Triton X-114 range between 45-99%.
✔ Triton X-114 results in a high product recovery (>80%).
✔ Extraction processes provide a rapid separation that is easily scalable and can achieve high removal efficiencies, especially with high initial concentrations.
-
1-octanol
Endotoxin has been effectively removed from T4, HAP1, and F8 bacteriophages using 1-octanol with endotoxin removal efficiencies varying between 64-99.9%. Additional processing is required to remove any trace quantities of 1-octanol present in the aqueous phase because the presence of 1-octanol causes high background noises reacting with LAL reagents. Even though the solvent extraction technique gives high endotoxin removal from various therapeutics solutions, the product yield is significantly low varying between 30-60%. Endotoxin removal efficiencies using 1-octanol range between 64-99.9%.
Creative Biolabs has a variety of methods for endotoxin removal and we develop customized endotoxin removal protocols for your target samples. To assure the highest efficiency after professional evaluation, one method or combination of several methods will be employed. We are the best choice for you to remove endotoxin from your various samples and we guarantee that all instruments, water, reagents, and consumables used in the experiment are free of endotoxins, and the experiment is conducted in a cleanroom to ensure that low levels of endotoxins are returned to the sample. If you are interested in our extraction assay, please feel free to contact us or directly send us an inquiry.
References
-
Mason, S.; et al. Current technologies to endotoxin detection and removal for biopharmaceutical purification. Authorea. 2019.
-
Petsch, D.; et al. Endotoxin removal from protein solutions. J Biotechnol. 2000, 76(2-3): 97-119.
For Research Use Only | Not For Clinical Use


 Fig.1 Removal of endotoxins by two-phase extraction with Triton X-114. (Petsch, 2000)
Fig.1 Removal of endotoxins by two-phase extraction with Triton X-114. (Petsch, 2000)
 Download our brochure
Download our brochure