FLT3 Assay Portfolio Service
Structure
FMS-like tyrosine kinase 3 (FLT3), also known as fetal liver kinase-2 (Flk-2) or stem cell tyrosine kinase-1 (STK-1), is a type III receptor tyrosine kinase (RTK). The human gene is located on chromosome 13q12 and encodes a 993 amino acids protein of 110 kD. Glycosylation with complex carbohydrates leads to the active and membrane-bound 158 kDa form. FLT3 is composed of an immunoglobulin-like extracellular ligand-binding domain (ECD), a transmembrane domain (TMD), a juxtamembrane dimerization domain (JMD), and a highly conserved intracellular tyrosine kinase domain (TKD) interrupted by a kinase insert. FLT3 is characterized by the presence of five immunoglobulin-like motifs within their extracellular part. In the cytoplasm, the JMD extends and connects with the two kinase domain lobes (TK1 and TK2) that are linked by the activation loop.
 Fig.1 The structure of FLT3. (Annesley, 2014)
Fig.1 The structure of FLT3. (Annesley, 2014)
The Expression of FLT3
FLT3 is expressed in normal human bone marrow. FLT3 is predominantly expressed by hematopoietic progenitor cells and its ligand (FL) is expressed as a membrane-bound or soluble form by bone marrow stromal cells. The expression is lost or reduced during differentiation into mature lymphoid and myeloid cells. The FLT3 receptor is overexpressed on the majority of acute myeloid leukemia (AML) blasts, and FL stimulation enhances proliferation and reduces apoptosis. FLT3 is also expressed in the human brain, placenta, and testis, though its function in these tissues remains unclear.
FLT3 Activation and Signal Pathways
The inactive form of FLT3 is maintained by interactions between the juxtamembrane (JM) domain and the kinase domain. Upon binding of the bivalent ligand through the third Ig-like domain (D3), JM is released from the kinase domain and autophosphorylation on several tyrosine residues takes place. And then auto-phosphorylation induces multiple signaling cascades. FLT3 mainly signals through Ras/mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3-kinase/protein kinase B (PI3K/Akt) pathways, involved in proliferation and survival.
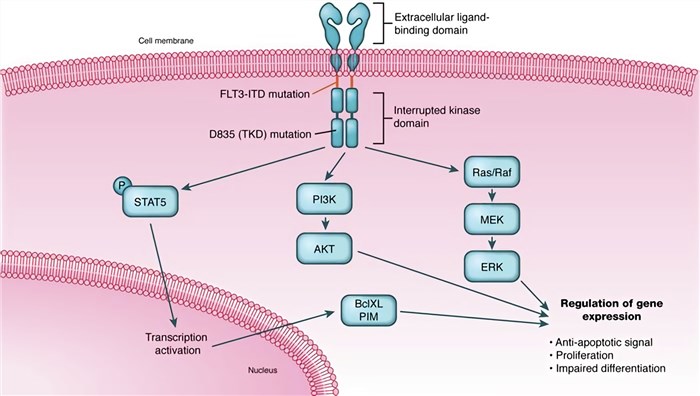 Fig.2 FLT3 mutations and signal pathways. (Simon, 2011)
Fig.2 FLT3 mutations and signal pathways. (Simon, 2011)
FLT3 Mutation
FLT3 mutations can be subdivided into two broad categories: the internal tandem duplications (ITD) within exon 14 and a single nucleotide polymorphism (SNP) that results from a point mutation in the activation loop of the TKD of FLT3.
-
FLT3-ITDs mutation.
The size of the duplication varies between three and more than 400 nucleotides, resulting in an elongation of the JMD between one and more than 130 amino acids. ITD mutations present in approximately 25% of AML patients and result in ligand-independent dimerization of the FLT3 receptor, constitutive phosphorylation, and activation of the kinase domains, culminating in cytokine-independent cellular proliferation. The specific mechanism by which FLT3/ITDs lead to auto-dimerization is unknown. FLT3/ITDs are thought to promote proliferation via the activation of multiple signaling pathways including RAS/MAPK, signal transducer and activator of transcription (STAT), and the AKT/PI3K pathways.
-
FLT3-TKD mutation.
The most frequent amino acid mutation in the TKD domain of FLT3 is the substitution of the Asp (D) at position 835 within the activation loop, which occurs in 7% of patients with AML. Mutation of D835V, D835Y, D839G, and I867S in TKD has been identified and characterized. All lead to receptor auto-activation.
The presence of FLT3-ITD mutations at diagnosis is predictive of a poor prognosis, associated with increased risk of relapse and reduced overall survival. The prognostic significance of FLT3-TKD is not clear, however, more complex, with reports of adverse effects, no effect, and a favorable prognosis. This discrepancy could be attributable to differences in mutant allelic burden.
What Can We Offer?
Most AML patients overexpress the wild-type FLT3 receptor in their blast cells. In addition, FLT3 mutations are common drivers of AML formation, identified in approximately one-third of newly diagnosed adult patients, and are common in pediatric AML as well. Because of the importance of FLT3 in tumors, Creative Biolabs provides a full set of FLT3 assay portfolio services for research, including but not limited to
-
FLT3-ITD gene mutation analysis
-
Cell proliferation assay
-
Cell apoptosis assay
-
In vitro kinase assay
-
Measurement of free intracellular calcium
-
Cell chemotaxis assay
-
Western blotting
-
Immunoprecipitation
Creative Biolabs is your expert to provide a large portfolio of tumor marker assay (e.g. FLT3 assay portfolio service). Our dedicated team of highly qualified scientists partners with external stakeholders to develop differentiated solutions that are fit-for-purpose. Please contact us to discuss your requirements.
References
-
Annesley, C.E.; et al. The Biology and targeting of FLT3 in pediatric leukemia. Front Oncol. 2014, 4: 263.
-
Simon, K.; et al. Emerging therapies for acute myeloid leukemia: translating biology into the clinic. Jci Insight. 2011, 2 (18): e95679.
For Research Use Only | Not For Clinical Use


 Fig.1 The structure of FLT3. (Annesley, 2014)
Fig.1 The structure of FLT3. (Annesley, 2014)
 Fig.2 FLT3 mutations and signal pathways. (Simon, 2011)
Fig.2 FLT3 mutations and signal pathways. (Simon, 2011)
 Download our brochure
Download our brochure