Human PBMC-T Mouse Model for Immuno-Oncology Study
Background
In the evolving landscape of immuno-oncology research, humanized mouse models have revolutionized our approach to understanding the interactions between tumors and the human immune system. Creative Biolabs, a pioneer in the field, offers cutting-edge platforms that integrate peripheral blood mononuclear cells (PBMCs) into immunodeficient mice, yielding an optimized and robust model for preclinical testing. Through the PBMC-T mouse model, researchers gain invaluable insights into immune-tumor dynamics, providing a more realistic simulation of human immune responses to both tumors and therapeutic interventions.
Human PBMC-T Mouse Model for Immuno-Oncology Study
The PBMC-T mouse model is an innovative tool wherein human PBMCs are transplanted into immunodeficient mice like NSG or NOD-SCID strains. This transplantation leads to the reconstruction of human-derived T cells, establishing a human immune microenvironment within the mice. Such a setting is crucial for evaluating the efficacy, pharmacodynamics, and mechanisms of action of diverse cancer therapeutics, including checkpoint inhibitors, monoclonal antibodies, therapeutic vaccines, and cytokine-based therapies.
Our Service Contents
-
Establishment of Humanized Mouse Model
Creative Biolabs offers comprehensive services for creating customized humanized mouse models tailored to specific research demands. The process includes:
Selection of the Mouse Background: Immunodeficient strains such as NSG or NOD-SCID are selected to ensure successful humanization.
Transplantation of Human PBMCs and Tumor Cells: Human PBMCs are transplanted into the mice, followed by the introduction of human tumor cells or tissues.
Pretreatment and Monitoring: The mice are pre-treated and monitored throughout the humanization process to ensure stable integration of human immune cells.
-
Immune Cell Detection and Monitoring
Advanced techniques like flow cytometry are employed to monitor and quantify the immune cells within the mouse post-transplantation. This includes:
Quantitative and Qualitative Analysis: Regular evaluations of the type and quantity of immune cells, ensuring accurate tracking of immune cell populations.
Functional Assessments: Assessing the functional status of these cells to verify the effective reconstruction of the human immune system.
Size Tracking: Tumor growth is consistently measured to observe changes over time.
Treatment Impact Evaluation: Analyzing how different therapeutic interventions affect tumor progression.
-
Immunotherapy Efficacy Evaluation
Our platform enables thorough assessments of various immunotherapy strategies:
Immune Modulation: Evaluating the therapeutic effects of immune checkpoint inhibitors, CAR-T cells, and other modalities.
Immune Cell Analysis: Before and after treatment, immune cells are analyzed using methods like flow cytometry to determine changes influenced by the therapy.
Highlights of Our Human PBMC-T Mouse Model
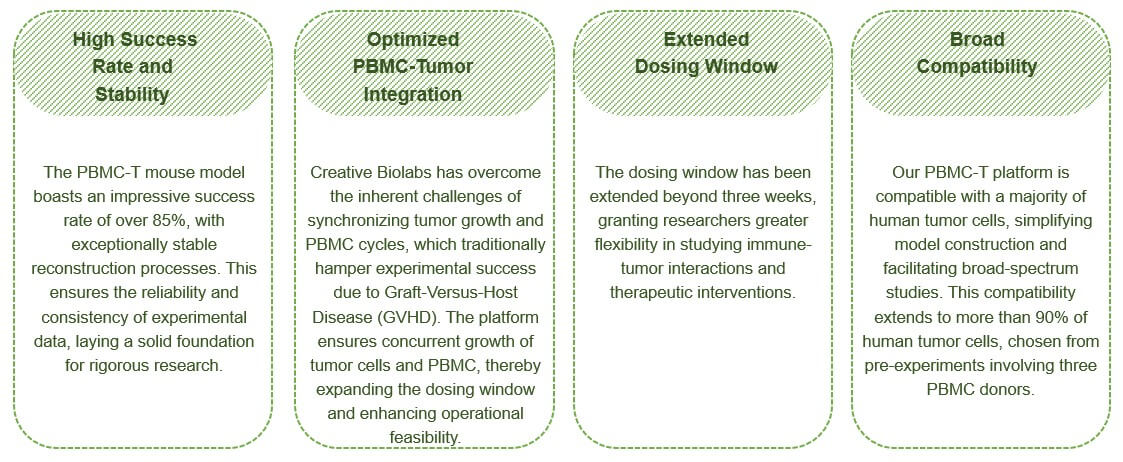
Frequently Asked Questions
Q1: What are the primary advantages of using the PBMC-T mouse model in immuno-oncology research?
A1: The PBMC-T mouse model offers a human immune microenvironment, stable reconstruction of human immune cells, extended dosing windows, and compatibility with a broad range of human tumor cells—all of which contribute to more accurate and reliable preclinical studies.
Q2: What is the significance of extending the dosing window in PBMC-T mouse models?
A2: Extending the dosing window beyond three weeks allows for more prolonged and flexible experimental timelines, enabling comprehensive studies of immune-tumor interactions and the evaluation of various therapeutic interventions.
By leveraging Creative Biolabs' innovative PBMC-T mouse models, researchers can gain a deeper understanding of immune-oncology dynamics, evaluate novel immunotherapies, and accelerate the development of effective cancer treatments.
For Research Use Only | Not For Clinical Use



 Download our brochure
Download our brochure