Oncolytic Virus Therapy Clinical Trials Overview
The progress made in cancer therapy with oncolytic viruses in preclinical studies has further encouraged the transition into clinical trials. Based on the real-time tracking of the virotherapy research, Creative Biolabs also provide up-to-date information and trend analysis of clinical trials.
Encouragingly, the first oncolytic therapeutic drug-Oncorine, based on oncolytic adenovirus type 5, was approved in China. Likewise, IMLYGIC (T-vector), a second-generation oncolytic herpes simplex virus-based drug for the treatment of melanoma, has been registered in the US and Europe as talimogene laherparepvec (Table 1).
Table 1 Approved Drugs of Oncolytic viruses
| Trade name | Virus | Genetic modifications | Therapeutic area | Company | Marketing start | Market |
| IMLYGIC® | HSV-1 | Deletions in ICP34.5 and ICP47 and transgenic expression of GM-CSF | Melanoma | Amgen, Inc. | October 27, 2015 |

|
| IMLYGIC® | HSV-1 | Deletions in ICP34.5 and ICP47 and transgenic expression of GM-CSF | Melanoma | Amgen, Inc. | December 16, 2015. |

|
| Oncorine | Human serotype 5 Ads | E1B-55KD and partial E3 deleted | Head and neck squamous cell carcinoma | Sunway Biotech | 2015 |

|
Oncorine
The first oncolytic virus approved by a regulatory agency was a genetically modified adenovirus named H101 by Shanghai Sunway Biotech, which gained regulatory approval in 2005 from China's State Food and Drug Administration (SFDA) for the treatment of head and neck cancer. Sunway's H101 and the very similar Onyx-15 have been engineered to remove a viral defense mechanism that interacts with a normal human gene p53, which is very frequently dysregulated in cancer cells. Despite the promises of early in vivo lab work, these viruses do not specifically infect cancer cells, but they still kill cancer cells preferentially. While overall survival rates are not known, short-term response rates are approximately doubled for H101 plus chemotherapy when compared to chemotherapy alone. It appears to work best when injected directly into a tumor, and when any resulting fever is not suppressed. Systemic therapy (such as infusion through an intravenous line) is desirable for treating metastatic disease. It is now marketed under the brand name Oncorine. Although subsequent clinical efficacy observed in human trials has failed to reach the high expectations based on studies in animal models, it remains the light of day for clinical trial workers.
IMLYGIC™
IMLYGIC™ (T-VEC/Talimogene Laherparepvec), a genetically engineered HSV, is the first OV approved in the United States and the European Union, indicated for the local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with melanoma recurrent after initial surgery. T-VEC is a genetically modified live oncolytic herpes virus therapy injected directly into melanoma lesions where T-VEC replicates inside the cancer cells to produce granulocyte-macrophage colony-stimulating factor (GM-CSF). The drug causes cell death and then rupture, releasing tumor-derived antigens and GM-CSF, which may promote an antitumor response. The properties and engineering sources are showed in table 2.
Table 2 Biology of T-VEC
| Property | Source |
| Selective TVEC replication and lysis in tumor cells. | Inherent suppression of PKR and type I interferon antiviral pathways in malignant cells. |
| Reduced neurovirulence with suppressed latency and ability to reactivate. | Deletion of both copies of the ICP34.5 neurovirulence gene. |
| Partial de-attenuation to enhance lytic activity in tumor cells. | Translocation of the US11 gene bringing it under an early promoter. |
| Enhanced antigen loading of MHC class I molecules. | Deletion of the ICP47 gene. |
| Dendritic cell accumulation and enhanced antigen presentation. | Insertion of 2 copies of the GM-CSF gene. |
Preclinical and clinical trials indicated that T-VEC had a strong ability to infect and drive transgene expression in a variety of cell types, preferentially lyse an array of human tumor cells, and induce antigen-specific CD8+ adaptive immune responses in both injected and uninjected tumors. Subgroup analysis of the OPTiM trial demonstrated a considerably higher durable response rate of 33% in stage III B/C patients and overall survival rate was significantly improved with TVEC for stage III B/C or IV M1a disease. The phase II trial reported objective response in 67% of injected lesions, 41% of injected non-visceral lesions, and only 13% of visceral lesions unavailable for injection. T-VEC is especially active against dermal satellite or in transit metastases. Thus, TVEC is highly effective at controlling locoregional disease, but systemic effects are limited and typically require combination approaches. Combined with checkpoint inhibitors, T-VEC also reacts in unresectable disease as well as in an array of other malignancies.
Clinical Trials of Oncolytic Viruses
- Number of Clinical Trials Over the Years
The first clinical trial application for oncolytic therapy began in 2001, but little progress was made in the following years, until 2006, when the quest for oncolytic virotherapy began. In the following time, applications for clinical programs gradually began to rise. Encouragingly, IMLYGIC™, a genetically engineered HSV, was approved as the first OV drug in the United States and the European Union in 2015, which led researchers to realize potential value in this immunotherapy. Since 2015, the clinical applications of oncolytic therapy have developed rapidly, especially with the largest number of projects in 2017, which shows an enormous potential of this industry. Likewise, the applications of 2018 are worth expectation. (Figure 1)
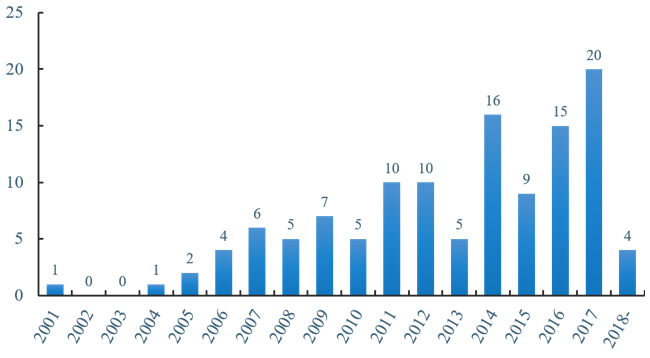 Figure 1 Number of clinical trials over the years
Figure 1 Number of clinical trials over the years
- Status of Lasted Clinical Trials of Oncolytic Virotherapy
There are currently 112 clinical trials listed on clinicaltrials.gov about oncolysis and oncolytic virus (July 2018). Of 112 clinical trials, 39 have been completed, 41 are in the process of recruitment, 18 are active but don't recruit, two have not yet recruited, and 12 have been withdrawn, suspended or terminated, or are otherwise unknown (Figure 2).
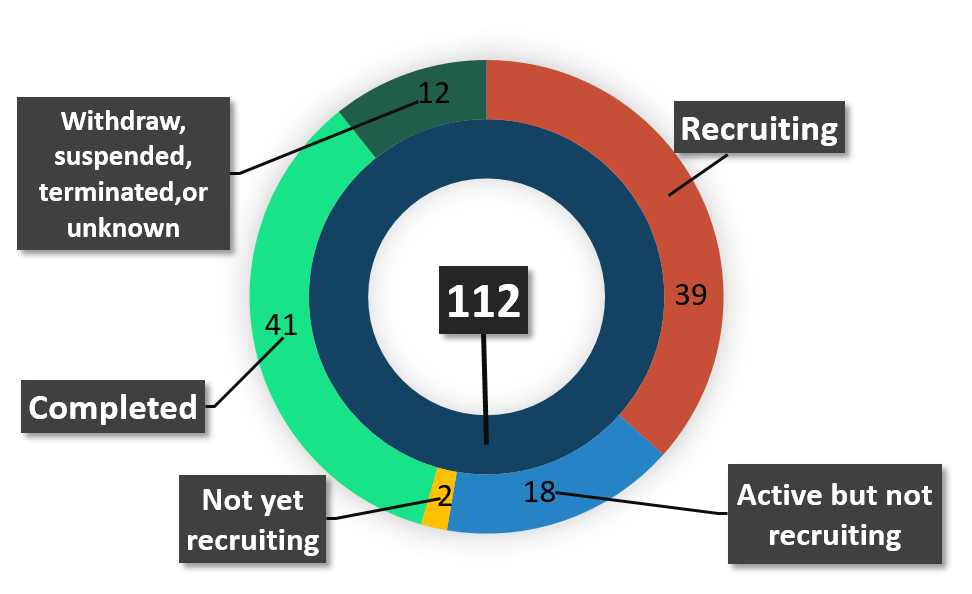 Figure 2 Status of lasted clinical trials of oncolytic Virotherapy
Figure 2 Status of lasted clinical trials of oncolytic Virotherapy
- Study Results of Effective Oncolytic Therapy Programs
Of the effective projects (completed, recruiting, active but not recruiting, not yet recruited), unfortunately, the 39 completed projects harvested only six test results while the rest encountered non-ideal data. And of the 61 ongoing projects, also only two yielded the results while the rest are still inconclusive. The results of clinical data showed that the clinical application of oncolytic virus is faced with both great challenges and opportunities (Figure 3).
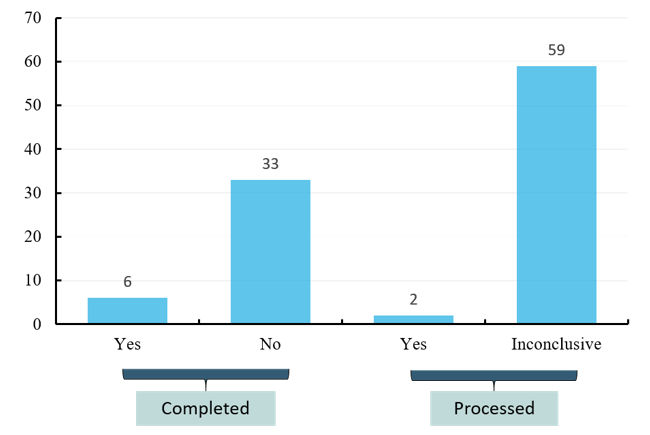 Figure 3 Study results of effective oncolytic therapy programs
Figure 3 Study results of effective oncolytic therapy programs
- Types of oncolytic viruses in clinical trials
Currently, more than ten oncolytic viruses approved by FDA have been involved in the clinical trials of cancers, most of which are engineering viruses. Human serotype 5 adenoviruss are associated with relatively mild diseases, and they are well characterized. Their genomes can be manipulated with relative ease, and they can be purified to high titer, hence acting as the most concerned engineered oncolytic virus. Herpes simplex virus is another of the most widely studied types, and recently much attention has also been paid to vaccinia virus and reovirus in recent years. (Figure 4)
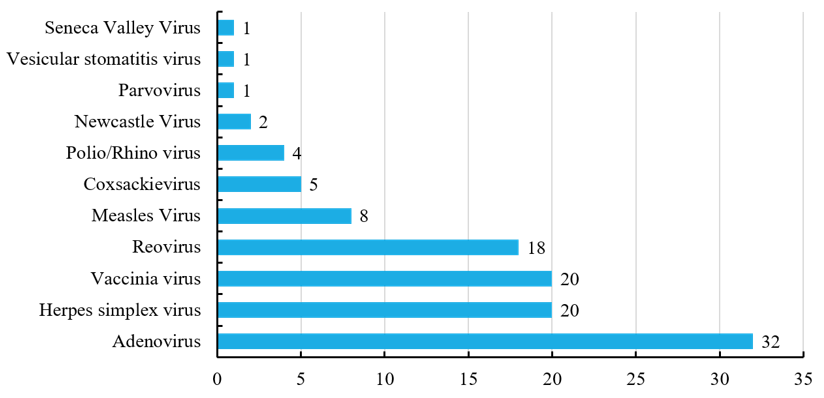 Figure 4 Types of oncolytic viruses in clinical trials
Figure 4 Types of oncolytic viruses in clinical trials
- Interventions of unfinished oncolytic therapies
Of those 61 currently unfinished projects, 32 are combination therapies of virus with some other modality, 16 of which are combined with antibody-based targeted immunotherapy, including pembrolizumab, tremelimumab, ipilimumab, bortezomib and rituximab, and the rest 27 test viruses by monotherapy. The data show that the combination of oncolytic virus and monoclonal antibody has great potential for treatment. (Figure 5)
Studies in animal models have confirmed that a combination approach gives the predicted improvement in therapeutic responses, such as the combination use of oncolytic viruses and other forms of cancer therapy, including cytotoxic chemotherapy, targeted molecular agents, radiation therapy and other forms of immunotherapy, such as cytokines, adoptive T cell therapy and, most notably, immune checkpoint inhibitors. Oncolytic virus-based combination strategies was further validated in early clinical trials, which reported increased objective response rates and tolerable safety profiles. While the efficacy of combination therapy is promising, data on the optimal dosing and scheduling of different treatment options are limited. There also lacks agreement about appropriate patient eligibility for oncolytic virus-based combination immunotherapy and study end points for monitoring the effectiveness of this approach.
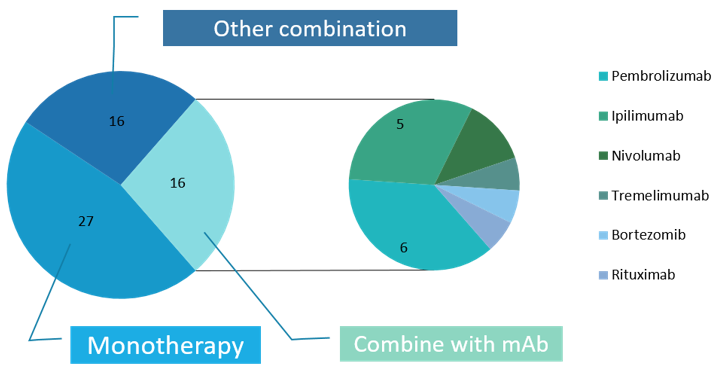 Figure 5 Interventions of recruiting, active, not recruiting and not yet recruiting oncolytic therapies
Figure 5 Interventions of recruiting, active, not recruiting and not yet recruiting oncolytic therapies
- Indications of oncolytic virotherapy in unfinished programs
Indications of oncolytic virotherapy show that the brain cancer is of various types, most of which are glioma cell cancers. Secondly, breast cancer, ovarian cancer, melanoma and pancreatic cancer have attracted widespread attention. Thanks to the efforts of scientists, oncolytic viral therapy has been largely developed in attempts to treat multiple cancers, aiming to achieve immune-boosting therapy (Figure 6).
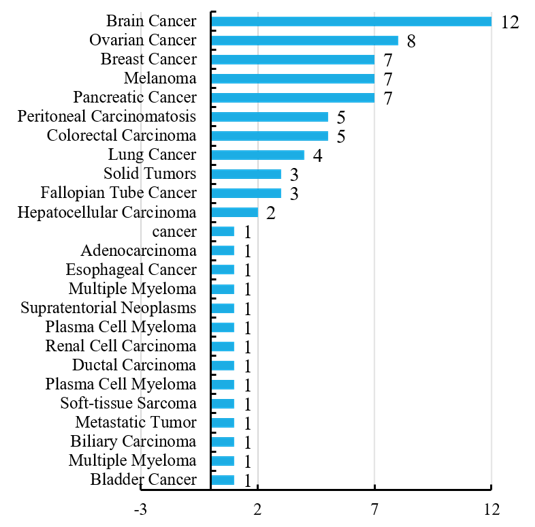 Figure 6 Indications of oncolytic virotherapy in unfinished programs
Figure 6 Indications of oncolytic virotherapy in unfinished programs
Although T-VEC remains the only oncolytic virotherapy approved by FDA at the time of this writing, clinical trials are underway with a wide variety of other modified viruses. And naturally occurring viruses have diverse tissue tropism and are more advantageous than others in different tumor histology. Thus, oncolytic virotherapy is a strategically attractive immunotherapy that is bound to rapid expansion in the near future to treat a wide array of malignancies both locoregionally and systemically by monotherapy and combination therapy.
Oncolytic viruses have shown efficacy in treating human tumors, and emerging clinical data support a role for oncolytic viruses in combination treatments with other cancer therapeutics, especially with other immunotherapy agents. Oncolytic viruses can affect almost all aspects of the cancer immunity cycle, and their antitumor activity occurs via multiple mechanisms, providing multiple opportunities for enhancing cancer immunotherapy.
Taking advantage of the OncoVirapy™ platform, Creative Biolabs provides customized, standardized, and reliable and high-quality oncolytic virus therapy development services for clients globally. We can make your research project incubate quickly and enter clinical trial smoothly.
