Serpin-2-based Tumor Selectivity Enhancement of Oncolytic Vaccinia Virus
Vaccinia virus (VV) is a typical member of the poxvirus family and replicates in the cell cytoplasm. It is an effective vaccine candidate and has been designed as a vaccine carrier for a variety of infectious diseases and malignant tumors. VV has several gene families, one of which encodes three proteins associated with serine protease inhibitors. The third serpin gene, called cytokine response modifier A (crmA) in cowpox virus (CPV) strain Brighton Red (BR), or B13R in VV Western Reserve (WR), collectively called serpin-2 (SPI-2), has been extensively studied.
Based on our comprehensive oncolytic virus development platform OncoVirapy™, Creative Biolabs is dedicated to providing the best-quality serpin-2 based tumor selectivity enhancing service to meet global customers' specific requirements.
Brief Introduction of Serpin-2
VV contains approximately 200 genes in its genome. They encode proteins to inhibit host immunity by interfering with the inflammatory response, like serine protease inhibitor (serpin) homologs SPI-2 and B22R. SPI-2 is a nonglycosylated peptide with a size of 38.5 kDa. It is expressed in an early phase of infection. SPI-2 shares 92% of its amino acid sequence with the cowpox virus modifier of the cytokine response known as crmA.
SPI-2 belongs to the superfamily of the inhibitors of serine proteases(serpins). Serpins are the most broadly distributed family of inhibitors of proteases. SPI-2 inhibits the proteolytic activity of caspase-1, also known as an interleukin-1β-converting enzyme(ICE), as well as granzyme B. Serpins are also involved in regulatory processes such as coagulation, cytokine activation, and antimicrobial peptides production. Moreover, SPI-2 expression inhibits the apoptotic pathway activated by Fas-ligand and TNFα. Both SPI-1 and SPI-2 genes of VV and rabbitpox were characterized as host range genes because the deletion of either gene exhibited host range defects.
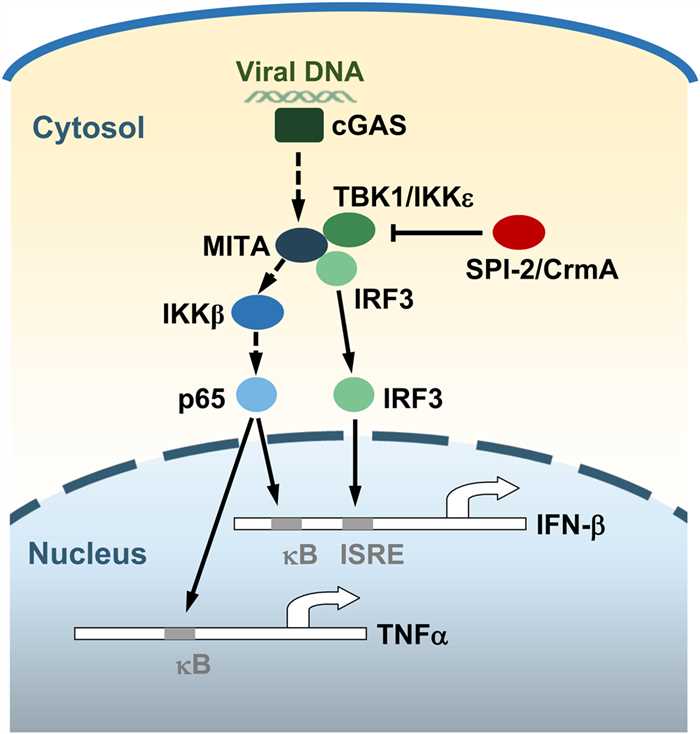 Fig.1 A model of the roles of SPI-2/CrmA in virus-triggered induction of downstream genes. (Qin, 2017)
Fig.1 A model of the roles of SPI-2/CrmA in virus-triggered induction of downstream genes. (Qin, 2017)
Impact on VV with the Deletion of SPI-2
In recent years, the VV has been widely concerned as a vector for tumor gene delivery and oncolytic virus therapy. It has been extensively studied for vaccine development. Importantly, it was observed that the deletion of SPI-2 leads to virus attenuation in the intranasal infection model but without any remarkable influence on the host immune response.
Scientists have shown that VV infection directly affects the mitochondrial apoptosis cascade by affecting the permeability transition pore, which can be shown by using the Copenhagen strain that naturally lacks the SPI-2 gene. In addition, the vSP, which was created by the deletion of SPI-2 in VV, retained high replication efficiency in cancer cells but significantly decreased in normal tissues. The research also suggests that the deletion of SPI-2 gene leads to the decrease of viral gene expression in some normal tissues while causing enhanced viral gene expression in tumor tissue. Importantly, vSP displayed even higher tumor selectivity in vivo. In conclusion, the impact on VV mediated by SPI-2 has important implications for rational vaccine design and antiviral drug development against VV infection.
With our well-established oncolytic virus engineering platform, Creative Biolabs is committed to delivering groundbreaking solutions to you.
Reference
- Qin, Y.; et al. SPI-2/CrmA inhibits IFN-β induction by targeting TBK1/IKKε. Sci Rep. 2017, 7: 10495.
