Therapeutic Transgene-loaded Oncolytic Vaccinia Virus
During the past decades, a phenomenal evolution of the oncolytic virus has been observed for many therapeutic applications. Creative Biolabs has established one-stop solutions to enhance the efficacy of oncolytic vaccinia virus (VACV) with the therapeutic transgene-loading strategy to meet the growing demands. In terms of our extensive experience in VACV development, our scientists are confident in providing VACV enhancement services for your specific projects.
Modified VACV with Enhanced Oncolytic Efficacy
- BMP4-loaded VACV
Glioblastoma multiforme (GBM) is the most aggressive malignant primary brain tumor in humans and generally has a poor prognosis. Evidence suggests that bone morphogenetic proteins (BMPs), which belong to the TGF-β superfamily of proteins, help regulate cancer in the context of GBM and induce rapid tumor regression. Tumor-targeted drug delivery is one of the major areas in cancer research, and viruses have already been used to deliver BMPs with good results. Therefore, expressing BMP payloads from a VACV platform has significant advantages in protein production and delivery in the tumor.
Researchers have developed an oncolytic vaccinia virus that overexpressed BMP4 and tested its activity in vitro and an orthotopic xenograft model of GBM. The virus overexpressing BMP4 promoted cell differentiation of primary GIC cultures derived from tumor biopsies, further increasing the replication capacity of the oncolytic virus. This study showed that inoculation of the BMP4-virus resulted in substantial tumor regression and survival benefit to mice. Furthermore, no tumor recurrence was observed in mice receiving BMP4-virus treatment, most likely due to the differentiation of cancer stem cells induced by virally expressed BMP4, which subsequently facilitates VACV replication and possibly depletes the cancer stem cell pool. These findings suggest that BMP4-VACV possesses enhanced oncolytic virotherapeutic potential against GBM in preclinical models.
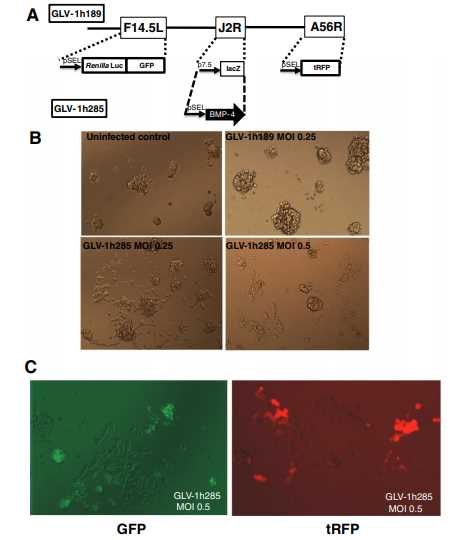 Fig.1 VACV expressing BMP-4 facilitates differentiation of GBM CSCs. (Duggal, 2013)
Fig.1 VACV expressing BMP-4 facilitates differentiation of GBM CSCs. (Duggal, 2013)
- rhEPO-loaded VACV
Recombinant human erythropoietin (rhEPO) is a glycoprotein hormone regulating red blood cell (RBC) formation and treating cancer-related anemia. Several studies have suggested that rhEPO also has antiapoptotic effects, induces angiogenesis, and promotes tumor growth. Researchers have reported the construction and characterization of the recombinant VACV, expressing rhEPO, and investigated the effects of rhEPO expressed locally in tumors. The results have shown that treatment with intravenous injection of VACV-rhEPO resulted in tumor-specific production and secretion of functional rhEPO, which exerted an effect on red blood cell progenitors and precursors. Moreover, mice receiving VACV-rhEPO exhibited enhanced tumor regression compared with mice injected with VACV alone. Therefore, VACV-mediated rhEPO expression not only enhanced oncolytic virotherapy but also simultaneously alleviated cancer-related anemia. It is conceivable that the combined benefits of VACV-rhEPO treatment, enhanced oncolytic activity, and systemic erythropoiesis could simultaneously improve treatment and ameliorate anemia in patients with cancer in clinical settings.
Therapeutic Antibody-loaded VACV for Enhancing Efficacy
- Anti-VEGF Antibody-loaded VACV
The replication-competent VACV is a promising candidate for oncolytic virotherapy of solid tumors in human tumor xenograft models in mice. Researchers have reported the construction of VACV strains encoding an engineered single-chain antibody (scAb) against both human and murine VEGFs for VACV-mediated and scAb-facilitated oncolytic virotherapy and immunotherapy in tumors. The VACV-encoded scAb exhibited significantly enhanced therapeutic efficacy compared with oncolytic viral therapy with the virus alone. Taken together, the VACV-mediated delivery and production of immunotherapeutic anti-VEGF scAb in tumors open the way for a unique therapy concept: tumor-specific and locally amplified drug therapy.
- Anti-TIGIT Antibody-loaded VACV
Although oncolytic virotherapy with VACV can lead to effective anti-tumor immunity by turning "cold" tumors into "hot" tumors, its therapeutic potential is affected by the tumor's local immunosuppressive tumor microenvironment (TME). Therefore, it is necessary to explore the use of immune checkpoint inhibitors to arm oncolytic VACVs to enhance their anti-tumor efficacy. Some researchers have generated a novel oncolytic VACV, VACV-α-TIGIT, in which the VACV is engineered to encode an anti-TIGIT mAb. This oncolytic VACV efficiently infected the tumor cells, replicated, lysed tumor cells, and secreted functional anti-TIGIT mAb. Intratumorally injection of VACV-α-TIGIT enhanced anti-tumor efficacy in several mice subcutaneous tumor models compared to VACV-Control. In conclusion, the engineered VACV with α-TIGIT is an effective strategy for oncolytic immunotherapy by combining viral oncolysis and intratumorally expression of immune checkpoint antibodies.
As a global pioneer leader in oncolytic VACV development, Creative Biolabs is professional in applying a series of leading technologies and services for our customers worldwide.
Reference
- Duggal, R.; et al. Vaccinia virus expressing bone morphogenetic protein-4 in novel glioblastoma orthotopic models facilitates enhanced tumor regression and long-term survival. Journal of translational medicine. 2013, 11(1), 1-15.
