Introduction to CD3
CD3 is a protein complex that constitutes a part of the T cell receptor (TCR) on the surface of T cells. It comprises four distinct polypeptide chains: CD3γ, CD3δ, CD3ε and CD3ζ, each containing one or more immunoreceptor tyrosine-based activation motifs (ITAMs). CD3 plays a crucial role in T cell activation and signal transduction by transmitting signals from the TCR to intracellular pathways such as Ras-MAPK, PI3K-Akt, and NF-κB. While primarily expressed in mature T cells, CD3 is also found in some immature thymocytes and natural killer (NK) cells. It represents a potential target for immunotherapy against various diseases and tumors, influencing the function and specificity of T cells. For instance, bispecific antibodies targeting CD3 and another antigen can redirect T cells to eliminate tumor cells or infected cells.
Introduction to CD19
CD19 is a protein forming part of the B cell co-receptor complex on the surface of B cells. Comprising a single transmembrane polypeptide chain with two extracellular immunoglobulin-like domains and a cytoplasmic tail with nine tyrosine residues, CD19 plays a pivotal role in B cell development, activation, and signal transduction. It enhances signals from the B cell receptor (BCR) to intracellular pathways like PLCγ2-Ca2+, PI3K-Akt, and NF-κB. While predominantly expressed on all stages of B cells except plasma cells, CD19 is a potential target for immunotherapy against various B cell-derived malignancies, including acute lymphoblastic leukemia (ALL), non-Hodgkin lymphoma (NHL), and multiple myeloma (MM). For instance, bispecific antibodies targeting CD19 and another antigen can recruit T cells or NK cells to eliminate tumor B cells or induce antibody-dependent cellular cytotoxicity (ADCC).
Signaling Pathways Involved in Bispecific Antibodies Targeting CD3 and CD19
Bispecific antibodies (BsAbs) are artificial antibodies with two different antigen-binding sites that can simultaneously target two different antigens or two different epitopes on the same antigen. BsAbs targeting CD3 and CD19 (BsAb-CD3/CD19) represent a novel form of immunotherapeutic drug developed using BsAb technology. These antibodies can recognize and connect CD3 on T cells and CD19 on B cells, achieving T cell-mediated B cell-specific killing. BsAb-CD3/CD19 initially binds to CD3 on T cells, causing phosphorylation of the ζ chain in the TCR/CD3 complex and activating downstream signal transduction molecules, such as ZAP-70, LAT, PLC-γ1, etc. These molecules further activate several signaling pathways, such as the Ras-MAPK pathway, the PI3K-Akt pathway, the NF-κB pathway, etc., leading to T cell activation, proliferation and cytokine release. At the same time, BsAb-CD3/CD19 binds to CD19 on B cells, causing CD19 phosphorylation and activating downstream signal transduction molecules such as Lyn, Syk, PI3K, etc., leading to B cell activation, proliferation and antibody secretion. Due to the mediation of BsAb-CD3/CD19, a tight immunological synapse forms between T cells and B cells, allowing T cells to induce B cell apoptosis by releasing effector molecules such as perforin and granzyme. In this way, BsAb-CD3/CD19 achieves a therapeutic effect on B cell-related diseases.
Clinical Status of Bispecific Antibodies Targeting CD3 and CD19
The clinical status of bispecific antibodies targeting CD3 and CD19 is promising, demonstrating significant efficacy and safety in various B cell-derived malignancies. To date, the FDA has approved only one bispecific antibody targeting CD3 and CD19, namely blinatumomab (Blincyto®). Blinatumomab, based on the bispecific T-cell engager platform, is a single-chain variable fragment (scFv) antibody consisting of two scFvs recognizing CD3 and CD19, respectively, connected by a short peptide without an Fc structure. FDA approval in 2014 covers its use in the treatment of relapsed or refractory B cell precursor acute lymphoblastic leukemia (B-ALL) and B cell precursor ALL in the first or second complete remission with minimal residual disease (MRD). Blinatumomab has also gained approval in Europe, Japan, and China.
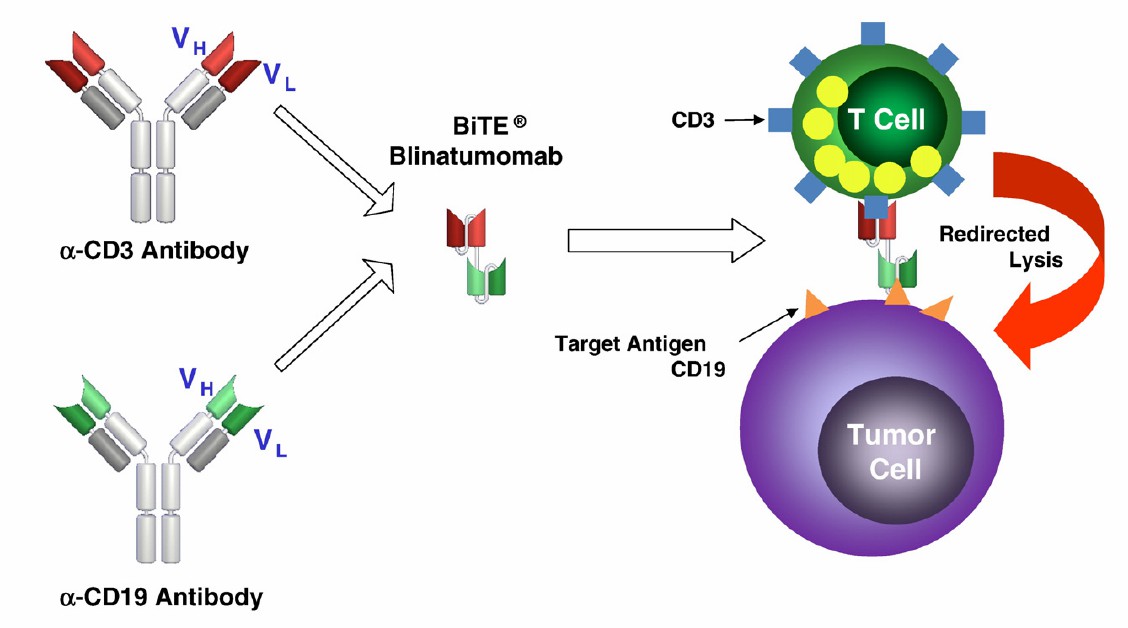
Fig.1 Blinatumomab: Structure and mechanism of action. (Nagorsen D, 2011)
Several other bispecific antibodies targeting CD3 and CD19 are currently in clinical trials, primarily developed by Roche/Genentech, Janssen, Merck, and other companies or research institutions. These antibodies are mainly intended for the treatment of B cell-derived malignancies, including B-ALL, non-Hodgkin lymphoma (NHL), and multiple myeloma (MM).
Table 1. Some of the bispecific antibodies targeting CD3 and CD19 in clinical trials
|
Drug
|
R & D institutions
|
Platform
|
Clinical Phase
|
Indications
|
|
MGD011
|
MacroGenics
|
Dual-Affinity Re-Targeting
|
II
|
B cell lymphoma
|
|
AFM11
|
Affimed
|
TandAbs
|
I
|
NHL
|
|
AMG562
|
Amgen
|
HLE-Bispecific T-Cell Engager
|
I
|
Lymphoma
|
|
A-319
|
Generon
|
ITab
|
I
|
ALL
|
References
1. Krishnamurthy A, Jimeno A. Bispecific antibodies for cancer therapy: A review. Pharmacol Ther. 2018 May;185:122-134.
2. Ma J, et al. Bispecific Antibodies: From Research to Clinical Application. Front Immunol. 2021 May 5;12:626616.
3. Wei J, et al. Current landscape and future directions of bispecific antibodies in cancer immunotherapy. Front Immunol. 2022 Oct 28;13:1035276.
4. Zuch de Zafra CL, et al. Targeting multiple myeloma with AMG 424, a novel anti-CD38/CD3 bispecific T-cell–recruiting antibody optimized for cytotoxicity and cytokine release. Clin Cancer Res. 2019 Jun 15;25(12):3698-3710.
5. Viardot A, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018 May 24;131(21):1522-1531.
6. Wu J, et al. Development of Novel Immunotherapies for Multiple Myeloma. Int J Mol Sci. 2019 Aug 23;20(17):4113.
7. Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017 Mar 2;376(9):836-847.
8. Topp MS, et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol. 2014 Dec 20;32(36):4134-4140.
9. Kantarjian H, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukaemia in the first salvage setting: a multicentre, open-label, randomised, phase 3 trial (TOWER). Lancet Oncol. 2020 Apr;21(4):494-503.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY










