Introduction of HER3
HER3 (Human Epidermal Growth Factor Receptor 3) is a receptor tyrosine kinase, also known as ERBB3 or ErbB-3, mainly expressed in epithelial cells, neuronal cells, and cardiomyocytes. Encoded by the ERBB3 gene, HER3 comprises an extracellular binding domain, a transmembrane domain, and an intracellular kinase domain. While its kinase activity is weak, it necessitates heterodimerization with receptors like HER2 to mediate signal transduction. Involved in various biological processes such as cell proliferation, differentiation, migration, and survival, abnormal HER3 expression or activation is associated with diverse tumors, making it a crucial target for cancer therapy.
Introduction of IGF-1R
IGF-1R (Insulin-like Growth Factor 1 Receptor), also known as CD221 or IGFIR, is a receptor tyrosine kinase expressed widely in various cells and encoded by the IGF1R gene. Comprising two α and two β subunits, with α subunits responsible for ligand binding and β subunits possessing kinase activity, IGF-1R mainly binds to growth factors IGF-1 and IGF-2, activating downstream PI3K/AKT and MAPK signaling pathways. IGF-1R regulates cell metabolism, proliferation, differentiation, migration, and apoptosis, and its overexpression or activation is associated with various malignant tumors, making it a promising target for cancer therapy.
Signaling Pathways Involved in Bispecific Antibodies Targeting HER3 and IGF-1R
Bispecific antibodies (BsAbs) targeting HER3 and IGF-1R represent a novel class of anti-tumor drugs capable of simultaneously blocking two signaling pathways, yielding synergistic or additive anti-tumor effects. As members of the ERBB and insulin receptor families, these receptors are frequently overexpressed or activated in various cancers, including gastric, esophageal, lung, breast, and ovarian cancers. BsAbs targeting HER3 and IGF-1R interfere with cross-talk and feedback mechanisms crucial in tumor progression and resistance to therapy.
BsAbs targeting HER3 and IGF-1R can exert their effects through different mechanisms, depending on their format and function. Some BsAbs can prevent the formation of heterodimers between HER3 and other ERBB family members (such as EGFR, HER2), thereby inhibiting the activation of downstream signaling pathways such as PI3K/AKT and MAPK/ERK. These pathways are responsible for regulating cell proliferation, survival, migration, and angiogenesis. Other BsAbs can prevent the binding of IGF-1R to its ligands (such as IGF-1 and IGF-2) or other receptors (such as insulin receptor), thereby inhibiting the activation of the same downstream signaling pathways. These ligands and receptors can also stimulate tumor growth, invasion, metastasis, and drug resistance.
In addition to blocking the signaling pathways, some BsAbs can also induce the internalization and degradation of HER3 and IGF-1R, thereby reducing their expression levels on the cell membrane. This can further impair the signaling capacity of these receptors and sensitize tumor cells to other therapies. Moreover, some BsAbs can also activate immune effector cells (such as natural killer cells and macrophages), killing tumor cells through antibody-dependent cellular cytotoxicity (ADCC) or antibody-dependent cellular phagocytosis (ADCP). This can enhance the anti-tumor immune response and overcome the immunosuppressive microenvironment.
Clinic Status of Bispecific Antibodies Targeting HER3 and IGF-1R
Currently, no approved BsAbs targeting HER3 and IGF-1R exist, but several are in clinical trials. For instance, isitarumab (MM-141) is a bispecific antibody targeting both HER3 and IGF-1R, inhibiting tumor cell growth and survival by blocking the signaling pathways of these two receptors. Clinical trials are underway for its potential application in treating malignant tumors such as pancreatic cancer and ovarian cancer. Isitarumab also enhances immune checkpoint inhibitor activity, activating T cell effector functions by depleting regulatory T cells.
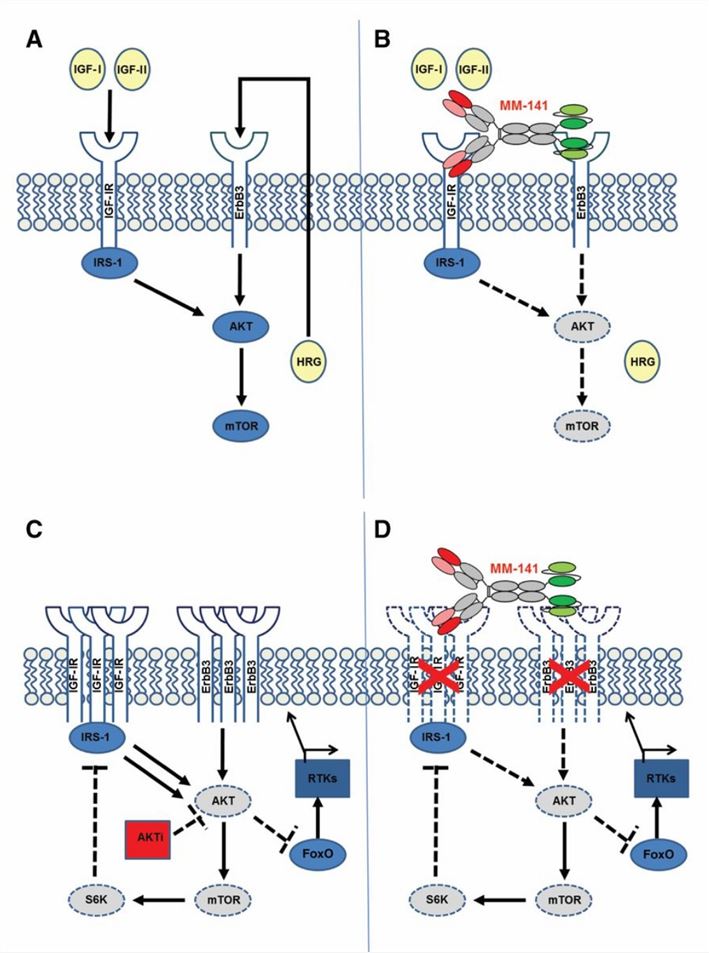
Fig.1 Model of MM-141 Mechanism of Action (Fitzgerald JB, 2014)
Table 1. Istiratumab (MM-141): A Bispecific Antibody Targeting IGF-1R and HER3
|
Property
|
Description
|
|
Type
|
Bispecific monoclonal antibody
|
|
Targets
|
IGF-1R and HER3 (also known as ErbB3)
|
|
Mechanism
|
Blocks ligand binding and induces receptor degradation through the proteasome pathway
|
|
Indications
|
Pancreatic cancer, ovarian cancer, and other malignancies
|
|
Development status
|
Phase II clinical trial completed
|
|
Developer
|
Merrimack Pharmaceuticals
|
References
1. Zhang N, et al. HER3/ErbB3, an emerging cancer therapeutic target. Acta Biochim Biophys Sin (Shanghai). 2016 Jan;48(1):39-48.
2. Geuijen CA, et al. Unbiased combinatorial screening identifies a bispecific IgG1 that potently inhibits HER3 signaling via HER2-guided ligand blockade. Cancer Discov. 2018 Jul;8(7):OF7.
3. Klinger M, et al. Bispecific antibodies: a new era in cancer immunotherapy. Curr Opin Immunol. 2016 Dec;40:14-20.
4. Spiess C, et al. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol. 2015 Oct;67(2 Pt A):95-106.
5. Schaefer W, et al. Immunoglobulin domain crossover as a generic approach for the production of bispecific IgG antibodies. Proc Natl Acad Sci U S A. 2011 Jul 5;108(27):11187-92.
6. Lewis SM, et al. Generation of bispecific IgG antibodies by structure-based design of an orthogonal Fab interface. Nat Biotechnol. 2014 Feb;32(2):191-8.
7. Fitzgerald JB, et al. MM-141, an IGF-IR- and ErbB3-directed bispecific antibody, overcomes network adaptations that limit activity of IGF-IR inhibitors. Mol Cancer Ther. 2014 Feb;13(2):410-25.
8. Camblin AJ, et al. Dual Inhibition of IGF-1R and ErbB3 Enhances the Activity of Gemcitabine and Nab-Paclitaxel in Preclinical Models of Pancreatic Cancer. Clin Cancer Res. 2018 Jun 15;24(12):2873-2885.
9. Kundranda M, et al. Randomized, double-blind, placebo-controlled phase II study of istiratumab (MM-141) plus nab-paclitaxel and gemcitabine versus nab-paclitaxel and gemcitabine in front-line metastatic pancreatic cancer (CARRIE). Ann Oncol. 2020 Jan;31(1):79-87.
10. Camblin AJ, et al. Dual targeting of IGF-1R and ErbB3 as a potential therapeutic regimen for ovarian cancer. Sci Rep. 2019 Nov 14;9(1):16832.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY










