Introduction of CD3
CD3 is a heteromeric protein complex comprised of four distinct subunits: gamma (CD3γ), delta (CD3δ), epsilon (CD3ε), and zeta (CD3ζ). These subunits, encoded by different genes on chromosome 11 in humans, each possess a single extracellular immunoglobulin-like domain, a transmembrane region with charged amino acids interacting with the TCR chains, and an intracellular tail with one or more immunoreceptor tyrosine-based activation motifs (ITAMs). Upon TCR stimulation, these ITAMs become phosphorylated. The CD3 complex associates either with the alpha/beta or gamma/delta heterodimers of the TCR, forming the TCR-CD3 complex expressed on the surface of all mature T cells.
Introduction of CD37
CD37, a member of the tetraspanin family of proteins, is characterized by four transmembrane domains, two extracellular loops, and short intracellular N- and C-termini. Encoded by the CD37 gene on chromosome 19 in humans, CD37 is expressed on the surface of mature B cells and B-cell malignancies, excluding plasma cells, T cells, NK cells, granulocytes, monocytes, or dendritic cells. CD37 can form homodimers or heterodimers with other tetraspanins, including CD53, CD81, and CD82. It also interacts with various membrane proteins, forming tetraspanin-enriched microdomains (TEMs) serving as platforms for signal transduction and cell-cell communication. CD37's main function is to regulate B-cell development, activation, adhesion, migration, and signaling.
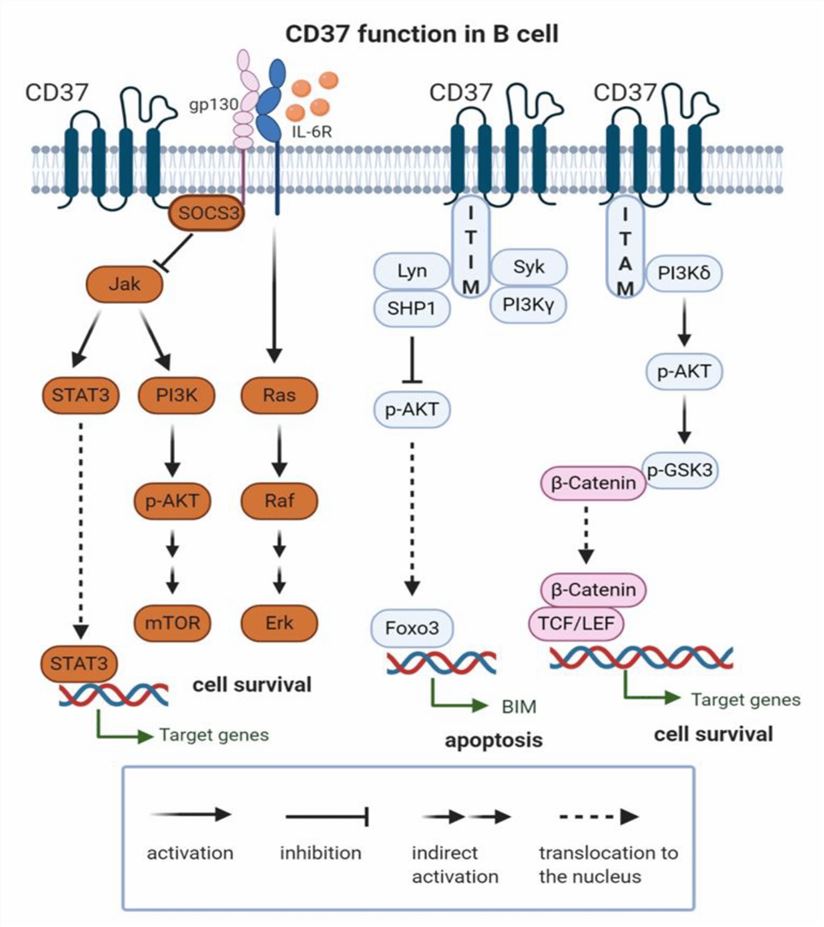
Fig.1 CD37 Function in B Cell (Bobrowicz M, 2020)
Signaling Pathways Involved in Bispecific Antibodies Targeting CD3 and CD37
BsAbs targeting CD3 and CD37 are designed to recruit and activate T cells against B-cell malignancies expressing high CD37 levels. These antibodies bind to both CD3 on T cells and CD37 on tumor cells, inducing the formation of immunological synapses between them. This activation leads to multiple signaling pathways in both cell types mediating their anti-tumor effects.
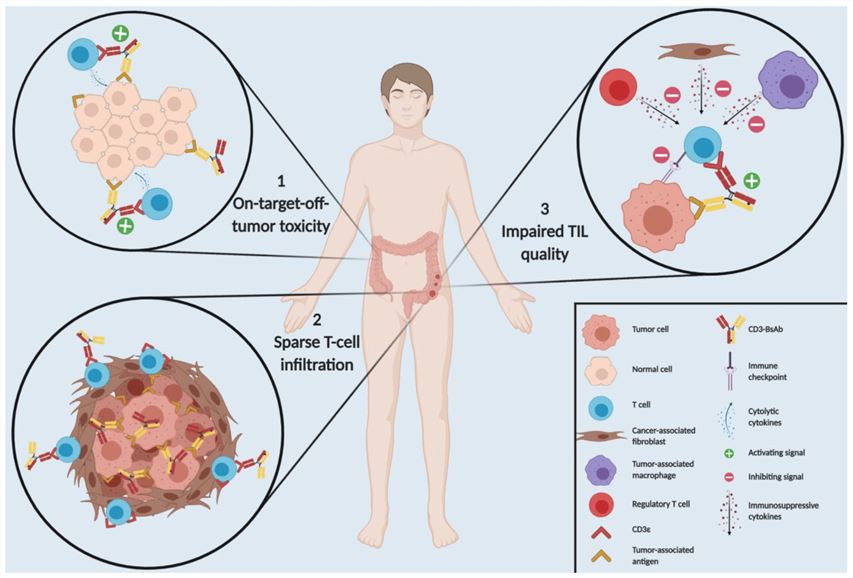
Fig.2 Three Main Hurdles for CD3-BsAb Therapy in Solid Tumors (Middelburg J, 2021)
In T cells, BsAbs binding to CD3 mimics antigen recognition by the TCR, triggering ITAM phosphorylation in CD3 subunits. This activation initiates downstream signaling molecules regulating T-cell activation, proliferation, differentiation, cytokine production, cytotoxicity, survival, and memory formation. Key signaling pathways include:
-
The Ras-Raf-MEK-ERK pathway, mediating activation of transcription factors such as AP-1 and Egr-1 regulating genes in T-cell proliferation and differentiation.
-
The PI3K-Akt-mTOR pathway, mediating activation of transcription factors such as NF-κB and FoxO regulating genes in T-cell survival, metabolism, growth, and function.
-
The PLCγ1-Ca2±NFAT pathway, mediating activation of transcription factors such as NFAT regulating genes in T-cell cytokine production and function.
In tumor cells, BsAbs binding to CD37 alters its interactions with other tetraspanins and membrane proteins, affecting signaling pathways regulating tumor cell survival, proliferation, migration, invasion, angiogenesis, and immune evasion. Key pathways include:
-
The BCR pathway: This pathway mediates the survival and proliferation of B-cell malignancies through various kinases such as Lyn, Syk, Btk, PI3K, Akt, ERK, etc. The binding of BsAbs to CD37 may interfere with this pathway by recruiting SHP-1 phosphatase that dephosphorylates these kinases.
-
The integrin pathway: This pathway mediates the adhesion and migration of B-cell malignancies through various integrins such as α4β1 (VLA-4), α5β1 (VLA-5), αLβ2 (LFA-1), etc. The binding of BsAbs to CD37 may interfere with this pathway by disrupting the interactions between integrins and their ligands such as VCAM-1 or ICAM-1.
-
The galectin pathway, mediating immune evasion of B-cell malignancies through galectins such as galectin-1 or galectin-3 binding to glycoproteins on tumor cells or immune cells. BsAbs binding to CD37 may interfere with this pathway by blocking interactions between galectins and their ligands such as CD45 or MHC class II.
References
1. Middelburg J, et al. Overcoming Challenges for CD3-Bispecific Antibody Therapy in Solid Tumors. Cancers (Basel). 2021 Jan 14;13(2):287.
2. Bobrowicz M, et al. CD37 in B Cell Derived Tumors-More than Just a Docking Point for Monoclonal Antibodies. Int J Mol Sci. 2020 Dec 15;21(24):9531.
3. Witkowska M, et al. Investigational therapies targeting CD37 for the treatment of B-cell lymphoid malignancies. Expert Opin Investig Drugs. 2018 Feb;27(2):171-177.
4. Langbein T, et al. Future of Theranostics: An Outlook on Precision Oncology in Nuclear Medicine. J Nucl Med. 2019 Sep;60(Suppl 2):13S-19S.
5. Saber H, et al. An FDA oncology analysis of CD3 bispecific constructs and first-in-human dose selection. Regul Toxicol Pharmacol. 2017 Nov;90:144-152.
6. Diebolder CA, et al. Complement is activated by IgG hexamers assembled at the cell surface. Science. 2014 Mar 14;343(6176):1260-3.
7. de Jong RN, et al. A novel platform for the potentiation of therapeutic antibodies based on antigen-dependent formation of IgG hexamers at the cell surface. PLoS Biol. 2016 Jan 7;14(1):e1002344.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY











