Introduction of PD1
PD1, also known as PDCD1 or CD279, stands for Programmed cell death protein 1. It is a protein found on the surface of T cells, B cells, NK cells, and other immune cells. PD1 is encoded by the PDCD1 gene on human chromosome 2. Its structure consists of a signal peptide, extracellular region, transmembrane region, and intracellular region. PD1 plays a crucial role in regulating T cell activation and tolerance by binding to its ligands PD-L1 and PD-L2. This helps maintain the balance of the immune system. PD1 is a potential target for treating various tumors and autoimmune diseases, as it can be exploited by tumor cells or inflammatory tissues to evade immune surveillance or suppress immune responses.
Introduction of CTLA4
CTLA4, also known as CD152, stands for Cytotoxic T-lymphocyte-associated protein 4. It is a protein found on the surface of activated T cells and regulatory T cells. CTLA4 is encoded by the CTLA4 gene on human chromosome 2. Its structure consists of a signal peptide, extracellular region, transmembrane region, and intracellular region. The main function of CTLA4 is to inhibit the costimulatory signal of T cells by binding to its ligands B7-1 and B7-2, which are also ligands for CD28, a stimulatory receptor on T cells. CTLA4 acts as an "off" switch for T cell activation, limiting their proliferation and differentiation. CTLA4 is a potential target for treating various tumors and autoimmune diseases, as it can be exploited by tumor cells or regulatory T cells to resist immune attack or induce immune tolerance.
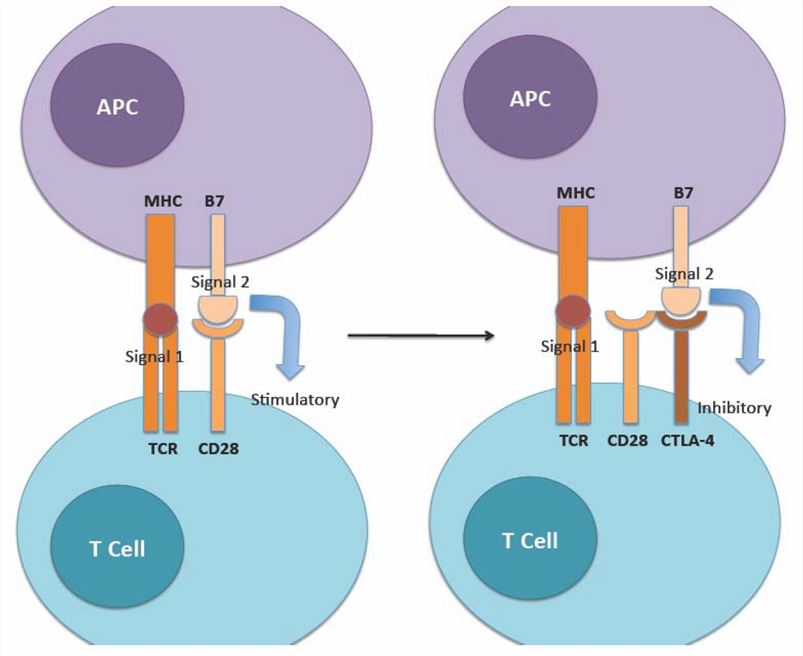
Fig.1 The physiologic role of CTLA-4 is to regulate the amplitude of the early stages of T cell activation (Longoria TC, 2015)
Signaling Pathways Involved in Bispecific Antibodies Targeting PD1 and CTLA4
Bispecific antibodies are antibody molecules that can recognize two different targets simultaneously. They can exert antitumor effects through various mechanisms. Bispecific antibodies that target PD1 and CTLA4 are a novel type of immune checkpoint inhibitors. They aim to block two important negative regulatory signals of T cells simultaneously, thereby enhancing the killing ability of T cells against tumor cells. The main signaling pathways involved in bispecific antibodies that target PD1 and CTLA4 are as follows:
-
The PD1/PD-L1/PD-L2 pathway: When PD1 binds to its ligands PD-L1 or PD-L2, it activates the intracellular region of PD1, triggering a series of signal transduction events, including the inhibition of the PI3K/Akt, Ras/MAPK, and NF-κB pathways, which leads to apoptosis, exhaustion, differentiation impairment, and functional deficiency of T cells. Bispecific antibodies targeting PD1 can block the interaction between PD1 and its ligands, restoring the activity and efficacy of T cells.
-
The CTLA4/B7-1/B7-2 pathway: When CTLA4 binds to its ligands B7-1 or B7-2, it inhibits the costimulatory signal of T cells mediated by CD28, which is also a receptor for B7-1 and B7-2. CTLA4 acts as an "off" switch for T cell activation, limiting their proliferation and differentiation. Bispecific antibodies targeting CTLA4 can block the interaction between CTLA4 and its ligands, enhancing the expansion and differentiation of T cells.
Clinic Status of Bispecific Antibodies Targeting PD1 and CTLA4
So far, among the bispecific antibodies (BsAbs) targeting PD1 and CTLA4, only one has been approved by the FDA: MEDI5752, a monovalent BsAb that binds to PD1 and CTLA4 with high affinity and specificity. MEDI5752 was approved in 2022 for the treatment of advanced solid tumors in patients who have progressed on or are intolerant to prior anti-PD-(L)1 therapy. MEDI5752 is currently available in the USA, EU, and Japan. Additionally, there are currently some BsAbs targeting PD1 and CTLA4 that have entered the clinical trial stage, mainly developed by pharmaceutical companies or research institutions in the United States, Europe, and China. These BsAbs primarily target refractory or resistant solid tumors, such as melanoma, lung cancer, liver cancer, gastric cancer, etc.
Table 1. BsAbs Targeting PD1 and CTLA4 in Clinical Trials
|
BsAb
|
Developers
|
Clinical trial IDs
|
Clinical trial phases
|
Clinical trial types
|
Clinical trial objectives
|
|
MEDI5752
|
AstraZeneca/ MedImmune
|
NCT03219268
|
Approved in 2022
|
Single-arm, multicenter, open-label, dose-escalation/expansion
|
Safety, tolerability, pharmacokinetics, pharmacodynamics, preliminary antitumor activity
|
|
REGN5678
|
Regeneron Pharmaceuticals/ Sanofi
|
NCT03821935
|
I/II
|
Single-arm, multicenter, open-label, dose-escalation/expansion
|
Safety, tolerability, pharmacokinetics, preliminary antitumor activity
|
References
1. Longoria TC, Eskander RN. Immune checkpoint inhibition: therapeutic implications in epithelial ovarian cancer. Recent Pat Anticancer Drug Discov. 2015;10(2):133-44.
2. Dovedi SJ, et al. Design and Efficacy of a Monovalent Bispecific PD-1/CTLA4 Antibody That Enhances CTLA4 Blockade on PD-1+ Activated T Cells. Cancer Discov. 2021 May;11(5):1100-1117.
3. Burton EM, Tawbi HA. Bispecific Antibodies to PD-1 and CTLA4: Doubling Down on T Cells to Decouple Efficacy from Toxicity. Cancer Discov. 2021 May;11(5):1008-1010.
4. Liu J, et al. PD-1/PD-L1 Checkpoint Inhibitors in Tumor Immunotherapy. Front Pharmacol. 2021 Sep 1;12:731798.
5. Li J, et al. Bispecific antibodies targeting CTLA-4: game-changer immunotherapy for cancer treatment? Front Immunol. 2023 Jan 14;12:1155778.
6. Wang C, et al. Development of bispecific antibodies and their applications in tumor immunotherapy. J Hematol Oncol. 2020 Dec 7;13(1):167.
7. Zhao Y, et al. The development of bispecific antibodies and their applications in tumor immune escape. Exp Hematol Oncol. 2019 Mar 14;8:3.
8. Zhang Y, et al. Bispecific antibodies as a development platform for new concepts and treatment strategies. Int J Mol Sci. 2017 Jan 6;18(1):48.
9. Kontermann RE, Brinkmann U. Bispecific antibodies. Drug Discov Today. 2015 Jun;20(7):838-847.
10. Spiess C, et al. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol. 2015 Oct;67(2 Pt A):95-106.
11. Labrijn AF, et al. Therapeutic bispecific antibodies: the next generation of immunotherapies? Expert Opin Biol Ther. 2019 Dec;19(12):1337-1350.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY










