CD3, a transmembrane protein complex, intricately associates with the T cell receptor (TCR), facilitating signal transduction upon antigen recognition. Comprising distinct chains—CD3γ, CD3δ, CD3ε, and CD3ζ—CD3 harbors immunoreceptor tyrosine-based activation motifs (ITAMs) in its intracellular domains. These ITAMs, when phosphorylated by kinases, recruit downstream signaling molecules like ZAP70 and LAT. Ubiquitously expressed on mature T cells and specific subsets of natural killer (NK) cells, CD3 stands as a promising target for cancer immunotherapy, as it activates T cells, inducing cytotoxicity against tumor cells expressing specific antigens.
Introduction of CD38
CD38, a type II transmembrane glycoprotein, serves diverse functions, including enzymatic activity, cell adhesion, and signal transduction. Functioning as an enzyme, CD38 catalyzes the synthesis and degradation of cyclic ADP-ribose (cADPR), a second messenger modulating intracellular calcium levels. As a cell adhesion molecule, CD38 interacts with CD31, expressed on platelets and endothelial cells. Acting as a signaling molecule, CD38 transduces signals through its cytoplasmic tail or cross-linking with other receptors like the B cell receptor (BCR) or the TCR. Widely expressed on hematopoietic cells and highly expressed on plasma cells and associated malignant cells, CD38 is a promising target for cancer immunotherapy, mediating antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), or direct apoptosis of tumor cells.
Signaling Pathways Involved in Bispecific Antibodies Targeting CD3 and CD38
BsAbs targeting CD3 and CD38 endeavor to redirect T cells to eliminate tumor cells expressing CD38. The mechanism encompasses several signaling pathways. The first pathway is the TCR-CD3 signaling pathway, which is triggered by the binding of the BsAb to the CD3 chain on the T cell surface. This leads to the phosphorylation of the ITAMs in the CD3ζ chain by the Src family kinase Lck. The phosphorylated ITAMs then recruit ZAP70, which activates downstream signaling cascades involving PLCγ1, IP3, DAG, PKCθ, NF-κB, MAPKs, and Ca2+. These signals induce the expression of activation markers (such as CD69 and CD25), cytokines (such as IL-2 and IFN-γ), and cytotoxic molecules (such as perforin and granzyme B) on the T cell. The second pathway is the cADPR-Ca2+ signaling pathway, which is initiated by the binding of the BsAb to the CD38 molecule on the tumor cell surface. This activates the enzymatic activity of CD38, which converts NAD+ to cADPR. cADPR then binds to ryanodine receptors (RyRs) on the endoplasmic reticulum (ER) membrane and releases Ca2+ into the cytosol. The elevated Ca2+ level triggers various cellular responses, such as apoptosis, autophagy, or differentiation. The third pathway is the Fas-FasL signaling pathway, which is mediated by the interaction of FasL on the activated T cell with Fas on the tumor cell. This leads to the formation of the death-inducing signaling complex (DISC), which activates caspase-8 and initiates the extrinsic apoptotic pathway.
Clinic Status of Bispecific Antibodies Targeting CD3 and CD38
BsAbs targeting CD3 and CD38 are in early clinical development for hematological malignancies, particularly multiple myeloma (MM) and acute leukemia. While no FDA-approved BsAb targeting CD3 and CD38 exists, several are undergoing clinical trials or are reported in preclinical studies. These BsAbs vary in formats, valencies, affinities, and pharmacokinetics.
Table 1. Selected BsAbs targeting CD3 and CD38 in Clinical Trials
|
Item
|
ISB 1342
|
Y150
|
|
Type
|
Bispecific antibody
|
Bispecific antibody
|
|
Target
|
CD38 and CD3 on multiple myeloma and T cells
|
CD38 and CD3 on multiple myeloma and T cells
|
|
Sponsor
|
Ichnos Sciences
|
Wuhan YZY Biopharma Co., Ltd.
|
|
Format
|
BEAT platform
|
YBODY platform
|
|
Status
|
Phase I clinical trial
|
Phase I clinical trial
|
|
Features
|
Low immunogenicity, alternative epitope to daratumumab, potent cytotoxicity
|
High affinity for both CD3 and CD38, low immunogenicity, potent cytotoxicity
|
|
NCT Number
|
NCT03309111
|
NCT05011097
|
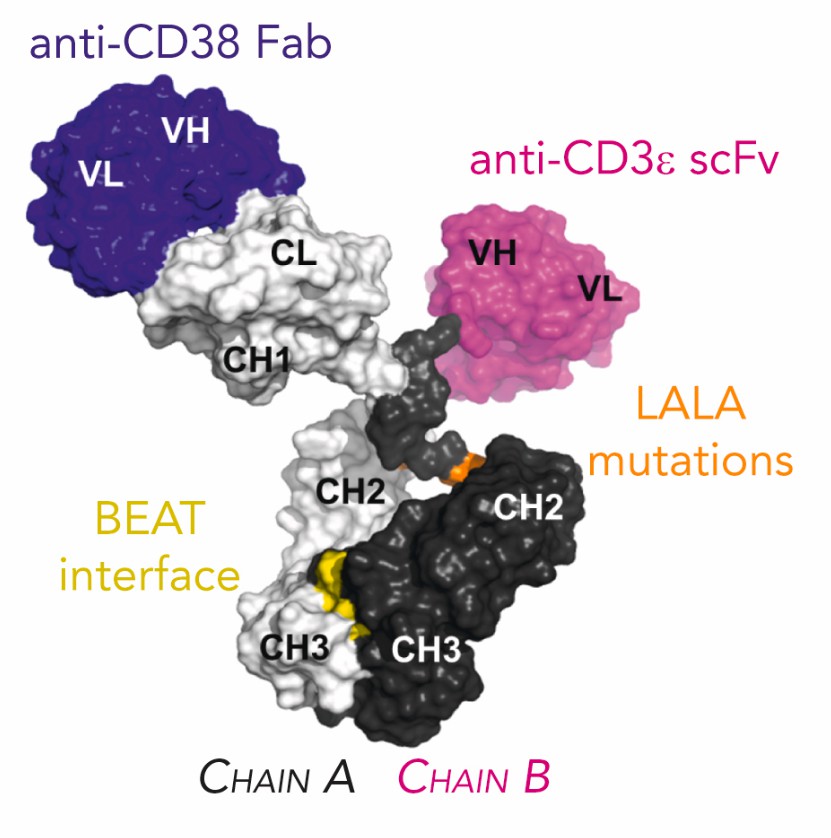
Fig.1 Schematic 3D Representation of ISB 1342 (Pouleau B, 2023)
References
1. Spiess C, et al. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol. 2015 Oct;67(2 Pt A):95-106.
2. Wu C, et al. Bispecific antibodies: a new class of therapeutics for hematological malignancies and beyond. Blood Rev. 2020 Sep;42:100648.
3. van der Veer MS, et al. The therapeutic potential of CD38-targeted immuno-therapies in multiple myeloma: from bench to bedside. Haematologica. 2016 Jul;101(7):784-91.
4. Seckinger A, et al. Target Expression, Generation, Preclinical Activity, and Pharmacokinetics of the BCMA-T Cell Bispecific Antibody EM801 for Multiple Myeloma Treatment. Cancer Cell. 2017 Mar 13;31(3):396-410.
5. Pouleau B, et al. Preclinical characterization of ISB 1342, a CD38 × CD3 T-cell engager for relapsed/refractory multiple myeloma. Blood. 2023 Jul 20;142(3):260-2731
6. Mohan SR, et al. Initial Results of Dose Escalation of ISB 1342, a Novel CD3xCD38 Bispecific Antibody, in Patients with Relapsed / Refractory Multiple Myeloma (RRMM). Blood. 2022 Nov 15;140(Supplement 1):7264-72662
7. Wu C, et al. Bispecific antibodies: a new class of therapeutics for hematological malignancies and beyond. Blood Rev. 2020 Sep;42:1006483
8. Wang Y, et al. A novel bispecific antibody targeting CD38 and CD3 induces potent cytotoxicity of autologous multiple myeloma cells in patients with relapsed or refractory multiple myeloma. Leukemia. 2020 Dec;34(12):3287-32974
9. Wang Y, et al. A novel bispecific antibody targeting CD38 and CD3 induces potent cytotoxicity of autologous multiple myeloma cells in patients with relapsed or refractory multiple myeloma: updated results from a phase I study [abstract]. In: Proceedings of the American Association for Cancer Research Annual Meeting 2021; April 10-15 and May 17-21, 2021; Philadelphia (PA): AACR; Cancer Res 2021;81(13 Suppl):Abstract nr CT183
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY










