Introduction of CD3
CD3 (cluster of differentiation 3) is a protein complex composed of four distinct subunits: CD3γ, CD3δ, CD3ε, and CD3ζ. These subunits are encoded by different genes located on chromosome 11 in humans. The CD3 complex associates with the TCR on the surface of T cells and transduces activation signals upon antigen recognition. The CD3 subunits contain immunoreceptor tyrosine-based activation motifs (ITAMs) in their cytoplasmic tails, which can be phosphorylated by Src-family kinases and recruit downstream molecules such as ZAP70 (zeta-chain-associated protein kinase 70) and LAT (linker for activation of T cells). These molecules then initiate various signaling cascades that lead to T cell proliferation, differentiation, cytokine production, and cytotoxicity. CD3 is expressed on all mature T cells, including CD4+ helper T cells, CD8+ cytotoxic T cells, regulatory T cells (Tregs), and γδ T cells. CD3 is also expressed on some subsets of natural killer (NK) cells and innate lymphoid cells (ILCs). CD3 is a potential target for immunotherapy of various cancers, as it can activate T cells against tumor antigens in an MHC-independent manner.
Introduction of CLEC12A
CLEC12A (C-type lectin domain family 12 member A) is a type II transmembrane glycoprotein that belongs to the C-type lectin-like receptor (CTLR) family. It is also known as CLL-1 (C-type lectin-like molecule-1), MICL (myeloid inhibitory C-type lectin-like receptor), DCAL-2 (dendritic cell-associated lectin 2), or CD371. CLEC12A is encoded by the CLEC12A gene located on chromosome 12 in humans. The extracellular domain of CLEC12A contains a single C-type lectin-like domain (CTLD) that can bind to carbohydrate structures such as mannose or fucose. The intracellular domain of CLEC12A contains an immunoreceptor tyrosine-based inhibitory motif (ITIM) that can recruit phosphatases such as SHP-1 (Src homology region 2 domain-containing phosphatase-1) and SHP-2 (Src homology region 2 domain-containing phosphatase-2). These phosphatases can dephosphorylate downstream signaling molecules and inhibit cellular activation. CLEC12A is expressed mainly on myeloid cells, including granulocytes, monocytes, macrophages, dendritic cells (DCs), and mast cells. CLEC12A is also expressed on AML blasts and LSCs, but not on normal HSCs. CLEC12A is a potential target for immunotherapy of AML, as it can selectively eliminate AML cells while sparing normal HSCs.
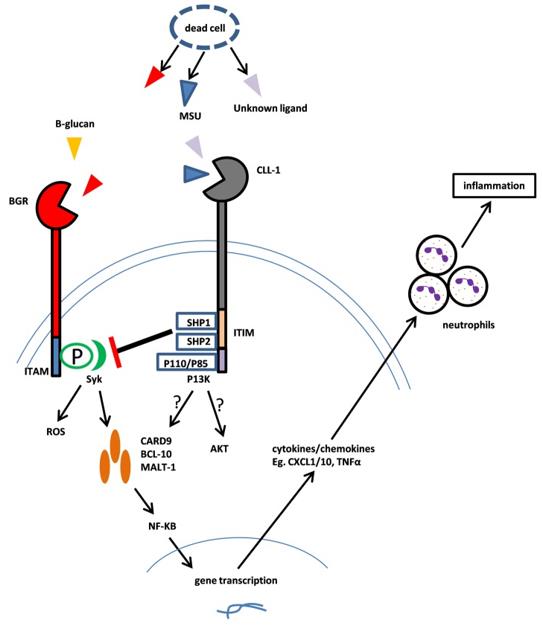 Fig.1 Mechanism of CLL-1 Function (Ma H, 2019)
Fig.1 Mechanism of CLL-1 Function (Ma H, 2019)
Signaling Pathways Involved in Bispecific Antibodies Targeting CD3 and CLEC12A
BsAbs targeting CD3 and CLEC12A can induce T cell-mediated cytotoxicity against AML cells by bridging the two antigens and forming an immunological synapse. The binding of BsAbs to CD3 triggers the phosphorylation of ITAMs in the CD3 subunits and the recruitment of ZAP70 and LAT. These molecules then activate several signaling pathways, such as the Ras-MAPK (mitogen-activated protein kinase), PI3K-Akt (phosphoinositide 3-kinase-Akt), and NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) pathways, that lead to T cell activation, proliferation, differentiation, cytokine production, and cytotoxicity. The binding of BsAbs to CLEC12A on AML cells may also modulate the signaling of CLEC12A by interfering with its interaction with its natural ligands or by inducing its internalization or downregulation. This may result in the inhibition of CLEC12A-mediated negative regulation of AML cell survival and proliferation. Furthermore, the binding of BsAbs to CLEC12A may also induce antibody-dependent cellular cytotoxicity (ADCC) or antibody-dependent cellular phagocytosis (ADCP) by engaging Fc receptors on NK cells or macrophages, respectively.
Clinic Status of Bispecific Antibodies Targeting CD3 and CLEC12A
To date, there is no BsAb targeting CD3 and CLEC12A that has been approved for clinical use. However, there are several BsAbs targeting CD3 and CLEC12A that are currently under clinical development for the treatment of AML. For example, MCLA-117 is a novel bispecific antibody that can target CD3 and CLEC12A, which has a CrossMab IgG1-like format, which means that it has an Fc region that can mediate effector functions such as ADCC and ADCP. MCLA-117 can bind to CD3 on T cells and CLEC12A on AML cells, and bring them into close proximity, resulting in the activation and expansion of T cells and the killing of AML cells. MCLA-117 has shown potent anti-leukemic activity in preclinical models and is currently being tested in phase I/II clinical trials in patients with relapsed or refractory AML or newly diagnosed AML.
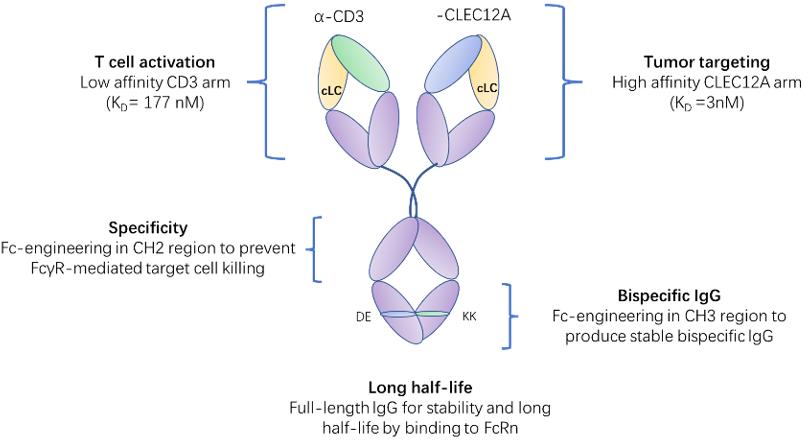 Fig.2 Schematic representation of MCLA-117 and the contribution of each domain to the functionality of MCLA-117 (Creative Biolabs)
Fig.2 Schematic representation of MCLA-117 and the contribution of each domain to the functionality of MCLA-117 (Creative Biolabs)
Table 1. Brief Overview of MCLA-117
|
Parameter
|
Description
|
|
Name
|
MCLA-117
|
|
Type
|
Bispecific antibody
|
|
Format
|
Full-length IgG1 with common light chain and silenced Fc region
|
|
Targets
|
CLEC12A and CD3
|
|
Mechanism of action
|
T cell-induced cytotoxicity of CLEC12A-positive leukemia cells
|
|
Developer
|
Merus N.V.
|
|
Clinical status
|
Phase I trial (MCLA-117-CL01) for relapsed or refractory AML patients
|
|
Clinical trial identifier
|
NCT02912274
|
|
Primary outcome measures
|
Safety, tolerability, maximum tolerated dose, recommended phase II dose, pharmacokinetics, pharmacodynamics, and preliminary efficacy
|
|
Secondary outcome measures
|
Minimal residual disease, overall survival, and duration of response
|
|
Estimated enrollment
|
120 participants
|
|
Estimated completion date
|
December, 2022
|
References
-
Middelburg J, et al. Overcoming Challenges for CD3-Bispecific Antibody Therapy in Solid Tumors. Cancers (Basel). 2021 Jan 14;13(2):287.
-
Singh A, et al. Overcoming the challenges associated with CD3+ T-cell redirection in cancer. Br J Cancer. 2021 Mar;124(6):1037-1048.
-
Morsink LM, et al. Prognostic and therapeutic role of CLEC12A in acute myeloid leukemia. Blood Rev. 2019 Mar;34:26-33.
-
Ma H, et al. Targeting CLL-1 for acute myeloid leukemia therapy. J Hematol Oncol. 2019 Apr 24;12(1):41.
-
Spiess C, et al. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol. 2015 Oct;67(2 Pt A):95-106.
-
Ma J, et al. Bispecific Antibodies: From Research to Clinical Application. Front Immunol. 2021 May 5;12:626616.
-
Kang J, et al. Immunotherapeutic progress and application of bispecific antibody in cancer. Front Immunol. 2022 Oct 20;13:1020003.
-
van Loo PF, et al. MCLA-117, a CLEC12AxCD3 bispecific antibody targeting a leukaemic stem cell antigen, induces T cell-mediated AML blast lysis. Expert Opin Biol Ther. 2019 Jul;19(7):721-733.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY


 Fig.1 Mechanism of CLL-1 Function (Ma H, 2019)
Fig.1 Mechanism of CLL-1 Function (Ma H, 2019)
 Fig.2 Schematic representation of MCLA-117 and the contribution of each domain to the functionality of MCLA-117 (Creative Biolabs)
Fig.2 Schematic representation of MCLA-117 and the contribution of each domain to the functionality of MCLA-117 (Creative Biolabs)







