Introduction of PDL1
PDL1, or programmed death-ligand 1 (CD274), is a transmembrane protein encoded by the CD274 gene, expressed on diverse immune and tumor cells, including dendritic cells, macrophages, T cells, B cells, and epithelial cells. PDL1 plays a pivotal role in regulating T cell activation and tolerance by binding to its receptors PD1 and CD80, expressed on T cell surfaces. This interaction results in the inhibition of T cell proliferation, cytokine production, and cytotoxicity, thereby suppressing the immune response against tumor cells. PDL1 expression correlates with poor prognosis and resistance to conventional therapies in various cancers like melanoma, non-small cell lung cancer, renal cell carcinoma, breast cancer, and gastric cancer. Targeting PDL1 with monoclonal antibodies has emerged as a promising strategy for cancer immunotherapy. However, challenges such as low response rates, immune-related adverse events, and primary or acquired resistance are associated with anti-PDL1 monoclonal antibodies.
Introduction of CTLA4
CTLA4, also known as cytotoxic T-lymphocyte-associated protein 4 or CD152, is a transmembrane protein encoded by the CTLA4 gene, expressed on activated T cells and regulatory T cells. CTLA4 plays a critical role in inhibiting T cell activation and proliferation by competing with CD28 for binding to CD80 and CD86, expressed on the surface of antigen-presenting cells. The interaction between CTLA4 and CD80 or CD86 attenuates T cell receptor signaling, induces T cell anergy, and promotes regulatory T cell function, preventing excessive immune activation and maintaining immune homeostasis. CTLA4 expression is linked to immune evasion and tumor progression in cancers such as melanoma, prostate cancer, colorectal cancer, and ovarian cancer. Targeting CTLA4 with monoclonal antibodies has emerged as a potent strategy for cancer immunotherapy, despite challenges like high toxicity, low response rates, and primary or acquired resistance.
Signaling Pathways Involved in Bispecific Antibodies Targeting PDL1 and CTLA4
Bispecific antibodies (BsAbs) are designed to simultaneously bind to two different targets, enhancing the specificity and efficacy of antibody therapy. BsAbs targeting PDL1 and CTLA4 block two crucial immune checkpoints, synergistically activating T cells against tumor cells. These BsAbs modulate signaling pathways involved in T cell activation and tumor cell survival. For instance, by blocking PD1/PDL1 interaction, BsAbs prevent the recruitment and activation of SHP-2, a phosphatase inhibiting downstream signaling pathways such as PI3K/AKT/mTOR, MAPK/ERK, and JAK/STAT, crucial for T cell proliferation, survival, differentiation, and cytokine production. Blocking CTLA4 with BsAbs prevents the competition of CTLA4 with CD28 for binding to B7 family co-stimulatory molecules like CD80 or CD86. This enhances the co-stimulatory signal of CD28, promoting activation of the same pathways. Moreover, BsAbs reduce the suppressive function of regulatory T cells (Tregs), which express high levels of CTLA4, inhibiting effector T cells through cell-cell contact or cytokine secretion. Simultaneously blocking PDL1 and CTLA4 with BsAbs enhances T cell activation, proliferation, cytokine secretion, cytotoxicity, induces tumor cell apoptosis and necrosis, and improves the tumor microenvironment.
Clinic Status of Bispecific Antibodies Targeting PDL1 and CTLA4
BsAbs targeting PD-1/PD-L1 and CTLA-4 represent a promising strategy to improve clinical outcomes in cancer immunotherapy, simultaneously blocking two crucial immune checkpoints and synergistically activating T cells against tumor cells.
Several other BsAbs targeting PD-1/PD-L1 and CTLA-4 are in various stages of clinical development. For example, KN046, a novel bispecific antibody simultaneously targeting PD-L1 and CTLA-4, was independently developed by Jiangsu Alphamab Biopharmaceuticals Co., Ltd., a subsidiary of Alphamab Oncology. KN046's unique design includes a proprietary CTLA-4 domain antibody with a significantly improved safety profile, a bispecific antibody fused with PD-L1 antibody, engineered to target the tumor microenvironment with high PD-L1 expression and Treg clearing function. KN046 has shown promising antitumor activity and tolerability in various solid tumors, such as non-small cell lung cancer, pancreatic cancer, hepatocellular carcinoma, and nasopharyngeal carcinoma. FDA clearance for phase II trials was granted based on clinical results in China and Australia. Additionally, KN046 received the U.S. FDA's orphan drug designation for thymic epithelial tumors in September 2020. Several pivotal clinical trials are ongoing, with the interim analysis of the phase III clinical study of KN046 combined with chemotherapy as the first-line treatment for non-small cell lung cancer successfully meeting the prespecified PFS endpoint.
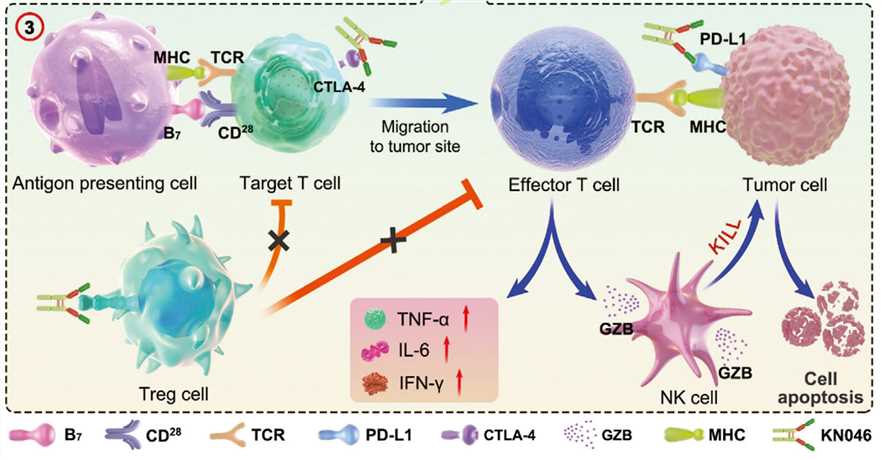
Fig.1 In vivo immune responses of dual-targeting inhibitor of immune checkpoints (Jiang C, 2021)
References
1. Jiang C, et al. Engineering a Smart Agent for Enhanced Immunotherapy Effect by Simultaneously Blocking PD-L1 and CTLA-4. Adv Sci (Weinh). 2021 Oct;8(20):e2102500.
2. Farhangnia P, et al. Bispecific antibodies targeting CTLA-4: game-changer troopers in cancer immunotherapy. Front Immunol. 2023 Jun 27;14:11557782.
3. Upadhaya S, et al. Challenges and opportunities in the PD1/PDL1 inhibitor clinical trial landscape. Nat Rev Drug Discov. 2022 Feb 10;21(2):137-1393.
4. Wang Y, et al. Phase I trial of KN046, a novel bispecific antibody targeting PD-L1 and CTLA-4, in patients with advanced solid tumors. J Immunother Cancer. 2021 Jun;11(6):e0066544.
5. Liddy N, et al. Design and Efficacy of a Monovalent Bispecific PD-1/CTLA4 Antibody That Enhances T Cell Activation and Saturation of CTLA4 without FcγR Binding or Toxicity. Cancer Discov. 2021 May;11(5):1100-11175.
6. Wang C, et al. Development of a PD-L1/CTLA-4 bispecific antibody for cancer immunotherapy. J Hematol Oncol. 2020 Oct 29;13(1):144.
7. Zhang Y, et al. A novel bispecific antibody targeting PD-L1 and CTLA-4 enhances antitumor immunity by reversing T cell exhaustion and suppressing regulatory T cells in vivo. Cancer Immunol Immunother. 2020 Sep;69(9):1827-1838.
8. Wang J, et al. A novel bispecific antibody targeting PD-L1 and CD47 induces tumor cell phagocytosis and exhibits potent antitumor efficacy in vivo. Biochem Biophys Res Commun. 2019 Jul 12;515(1):180-186.
9. Chen X, et al. A novel bispecific antibody targeting PD-L1 and CD47 enhances T cell activation and killing of tumor cells in vitro and in vivo. Biochem Biophys Res Commun. 2019 Jun 18;513(4):1040-1046.
10. Liu B, et al. Simultaneous Blockade of Both PD-L1 and CTLA-4 Enhances Antitumor Activity in Non-Small Cell Lung Cancer by Augmenting CD8+ T Cell Responses Mediated by NK Cells and Monocytes/Macrophages via IFNγ Signaling Pathway Modulation. Front Immunol. 2018 Nov 13;9:2625.
11. Liu B, et al. A Novel Bispecific Antibody Targeting PD-L1 and CTLA-4 Augments Tumor Antigen-Specific CD8+ T Cell Responses for Enhanced Antitumor Immunity both In Vitro and In Vivo. Front Immunol. 2018 Jul 31;9:1780.
12. Liu B, et al. A Novel Bispecific Antibody Targeting PD-L1 and CTLA-4 Enhances Antitumor Immunity by Inducing CD8+ T Cell Activation via IFNγ Signaling Pathway Modulation in Non-Small Cell Lung Cancer Cells In Vitro and In Vivo. Front Immunol. 2018 Jul 31;9:1779.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY










