Introduction of PD1
PD1 is the abbreviation for programmed cell death protein 1, also known as CD279. It is a transmembrane protein expressed on activated T cells, B cells, NK cells, and NKT cells. It is encoded by the PDCD1 gene. The PD1 protein consists of an extracellular domain, a transmembrane domain, and an intracellular domain. The main biological function of PD1 is to inhibit T cell receptor (TCR) signaling by binding to its ligands PD-L1 and PD-L2, regulating T cell activation and exhaustion states. The PD1/PD-L1 pathway plays an important role in various diseases, especially in tumor immune evasion, where tumor cells suppress the immune system's attack by expressing PD-L1. Therefore, antibodies targeting the PD1/PD-L1 pathway have become an effective tumor immunotherapy method.
Introduction of LAG3
LAG3 is the abbreviation for lymphocyte-activation gene 3, also known as CD223. It is a transmembrane protein expressed on activated T cells, B cells, NK cells, NKT cells, and regulatory T cells. It is encoded by the LAG3 gene. The LAG3 protein consists of an extracellular domain, a transmembrane domain, and an intracellular domain. The main biological function of LAG3 is to regulate T cell and other immune cell activation and tolerance by binding to its ligands MHC-II, FGL-1, α-synuclein, galectin-3, and LSECtin. LAG3 is an important immune checkpoint molecule that synergizes with PD1 to inhibit tumor-specific T cell function in tumor immune evasion. Therefore, antibodies targeting LAG3 or in combination with antibodies targeting PD1 have become a promising immunotherapy strategy for cancer.
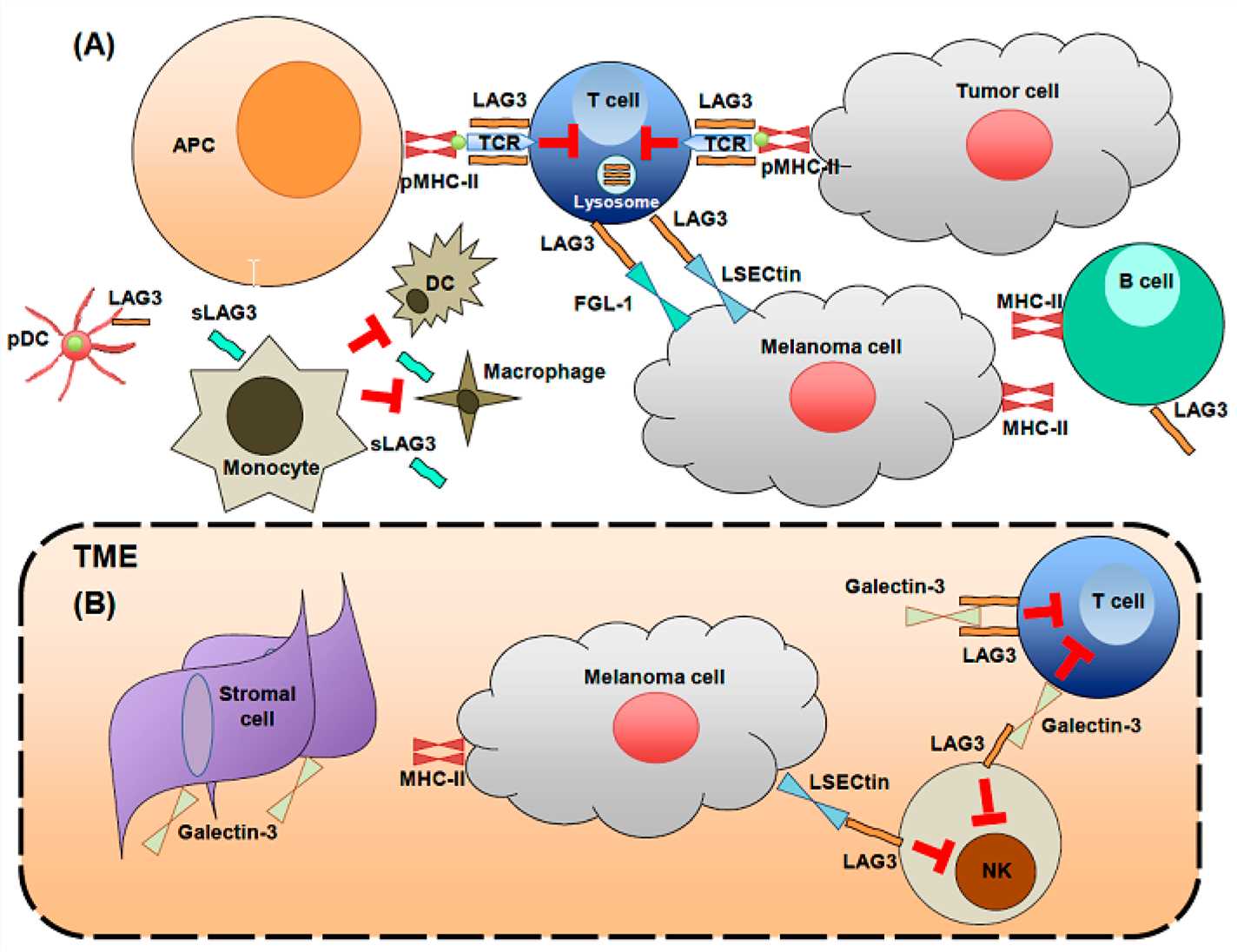
Fig.1 LAG3 biology on immune cells and in the tumor microenvironment (Solinas C, 2019)
Signaling Pathways Involved in Bispecific Antibodies Targeting PD1 and LAG3
One of the strategies to enhance the antitumor efficacy of PD-1/PD-L1 blockade is to combine it with other immune checkpoint inhibitors, such as LAG3. LAG3 is another inhibitory receptor that is expressed on activated T cells, regulatory T cells, NK cells, and dendritic cells. LAG3 binds to MHC-II molecules or FGL1 on antigen-presenting cells or tumor cells and suppresses T cell activation and proliferation. LAG3 also induces T cell tolerance and exhaustion. Therefore, blocking LAG3 can restore T cell function and potentiate the effect of PD-1/PD-L1 blockade.
Bispecific antibodies targeting PD-1 and LAG3 can simultaneously disrupt two inhibitory pathways and enhance T cell activation and function. When PD-1 binds to its ligands PD-L1 or PD-L2, it activates the PTEN/PI3K/AKT pathway, which leads to T cell apoptosis, metabolic dysfunction, and exhaustion. When LAG3 binds to its ligands MHC-II or FGL1, it activates the SHP-1/SHP-2 pathway, which leads to T cell proliferation inhibition, cytokine secretion reduction, and tolerance induction. Bispecific antibodies targeting PD-1 and LAG3 can interfere with both pathways and restore T cell vitality and efficacy.
Clinic Status of Bispecific Antibodies Targeting PD1 and LAG3
So far, no bispecific antibodies targeting PD1 and LAG3 have been approved for marketing. However, several bispecific antibodies targeting PD1 and LAG3 are currently in different stages of clinical trials, mainly developed by companies or research institutions such as MacroGenics, EpimAb Biotherapeutics, Roche, F-star Therapeutics, and Innovent Biologics. These bispecific antibodies mainly target solid tumors and hematological malignancies, including melanoma, breast cancer, non-small cell lung cancer, renal cell carcinoma, and so on. Among them, Tebotelimab (MGD013) is a PD1/LAG3 bispecific antibody based on DART technology, which has entered phase III clinical trial for the treatment of advanced solid tumors. EMB-02 is a PD-L1/LAG3 bispecific antibody based on FIT-Ig technology, which has entered phase II clinical trial for the treatment of advanced solid tumors. RG6139 is a PD-L1/LAG3 bispecific antibody based on CrossMab technology, which has entered phase II clinical trial for the treatment of advanced solid tumors. FS118 is a PD-L1/LAG3 bispecific antibody based on mAb² technology, which has entered phase II clinical trial for the treatment of cancer. IBI-323 is a PD-L1/LAG3 bispecific antibody based on Knob-into-Hole technology, which has entered phase I clinical trial for the treatment of cancer.
Table 1. Bispecific Antibodies Targeting PD1 and LAG3 in Clinical Trials
|
Antibody
|
Clinical stage
|
Indication
|
Country/region
|
|
Tebotelimab (MGD013)
|
II/III
|
Solid tumors and hematological malignancies
|
USA, China and so on
|
|
EMB-02
|
II
|
Solid tumors
|
USA, China and so on
|
|
RG6139 (RO7121661)
|
II
|
Solid tumors
|
USA, EU and so on
|
|
FS118 (F-star-118)
|
II
|
Cancer
|
USA, EU and so on
|
|
IBI-323 (Sintilimab/LAG-3)
|
I
|
Cancer
|
China
|
References
1. Solinas C, et al. LAG3: The Biological Processes That Motivate Targeting This Immune Checkpoint Molecule in Human Cancer. Cancers (Basel). 2019 Aug 20;11(8):1213.
2. Workman CJ, et al. Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J Immunol. 2004 Apr 15;172(8):5450-5.
3. Huang CT, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004 Oct;21(4):503-13.
4. Andrews LP, et al. LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev. 2017 Mar;276(1):80-96.
5. Woo SR, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012 Feb 15;72(4):917-27.
6. Ascierto PA, et al. Future perspectives in melanoma research: meeting report from the “Melanoma Bridge”: Napoli, December 5th-8th 2013. J Transl Med. 2014 May 9;12:137.
7. Brignone C, et al. A phase I pharmacokinetic and biological correlative study of IMP321, a novel MHC class II agonist, in patients with advanced renal cell carcinoma. Clin Cancer Res. 2009 Sep 15;15(18):6225-31.
8. Brignone C, et al. First-line chemoimmunotherapy in metastatic breast carcinoma: combination of paclitaxel and IMP321 (LAG-3Ig) enhances immune responses and antitumor activity. J Transl Med. 2010 Jul 9;8:71.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY










